Elements and atoms
What is an element?
The word “element” can be used in two ways:
- An element is one of the 118 different types of atoms e.g. Hydrogen, Helium, Neon etc. (we have arranged these in the periodic table of the elements).
- An element is a pure substance made of only one kind of atom (e.g. a lump of gold is an element)
What is the structure of atoms?
An atom is the smallest unit of an element.
Remember, in neutral atoms, there are equal numbers of protons and electrons.

What is atomic number and mass number?
The atomic number of an element is the number of protons present in each atom.
When atoms are electrically neutral (when they haven’t gained or lost electrons), the number of protons in an atom is the same as the number of electrons.
The mass number of an atom is the sum of the number of protons and neutrons in the atom. The number of neutrons in an atom can therefore be calculated by subtracting the atomic number from the mass number.
What is the atomic number and mass number of the atom shown?
How to work out the number of neutrons?
Once you know the atomic number and the mass number of an element, you can work out how many electrons and neutrons are in that element.
For example, the atom of iron shown below has a mass number of 56 and an atomic number of 26.

To calculate the number of neutrons, the atomic number (number of protons) is subtracted from the mass number (protons + neutrons) to give 30 neutrons:
number of neutrons = Mass number − Atomic number = 56 − 26 = 30
What do the numbers next the atomic symbols mean?

What was Rutherford’s model?
As a result of his experiments, Rutherford had to propose a new model of the atom.
This model has since been changed in light of new experiments and evidence.
Why did Bohr change the model of the atom?
Early models of the atom could not explain the emission of particular wavelengths of light by the atoms of elements under certain conditions.
For example, when gain energy from heat or electricity, they can emit light of particular colours.

We can do experiments called flame tests to show this!
What are emission spectra?

Atoms of a gas can emit light of certain colours when energy is supplied to the gas, either by heating (e.g. flame tests) or when atoms in a gas are excited after a potential difference is applied.
The characteristic colours are called an emission spectrum and appear as lines when viewed through a prism.
The image shows the “visible” hydrogen emission spectrum.
One of the problems with the Rutherford model is that it was unable to explain the emission spectrum of Hydrogen (or any other element).
The emission spectrum is an observation which demands an explanation!
What is Bohr’s Model of the Atom?
To overcome difficulties associated with the Rutherford model of the atom, including the challenge of explaining the emission spectrum (the light emitted during flame tests) Niels Bohr proposed a new model.
Bohr extended Rutherford’s atomic model by arranging the electrons in concentric spherical shells. The electrons orbit the nucleus in classical circular paths.
Bohr proposed that electrons could orbit the nucleus in a stable manner only at a few specific distances from the nucleus whereas all other orbital radii were unstable.
The electrons can only occupy the shells and cannot exist between shells.
How did Bohr’s model explain the emission spectrum?
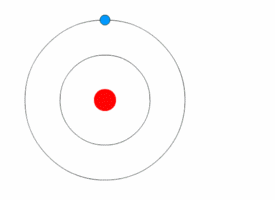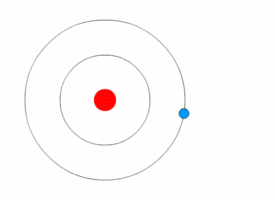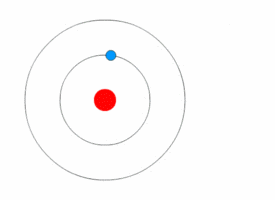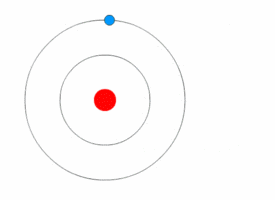
Each electron shell is an energy level. In other words, the electrons in each shell have a certain amount of energy.
When electrons move between shells, they emit or absorb electromagnetic radiation (often visible light).
Because the electrons can only have specific energy levels, they can only absorb and emit light of certain wavelengths.
Electron configuration
Each electron shell can only hold a certain number of electrons.
The innermost shell is the smallest and is filled with electrons first, followed by the outer shells.
The shells are numbered 1, 2, 3 etcetera. These numbers correspond to the principal quantum numbers (n = 1, 2, 3, 4 …)
Shells can also be labeled alphabetically with the letters K, L, M, … Your booklet does this in places.
How are the electrons arranged in Bohr’s model of the atom?
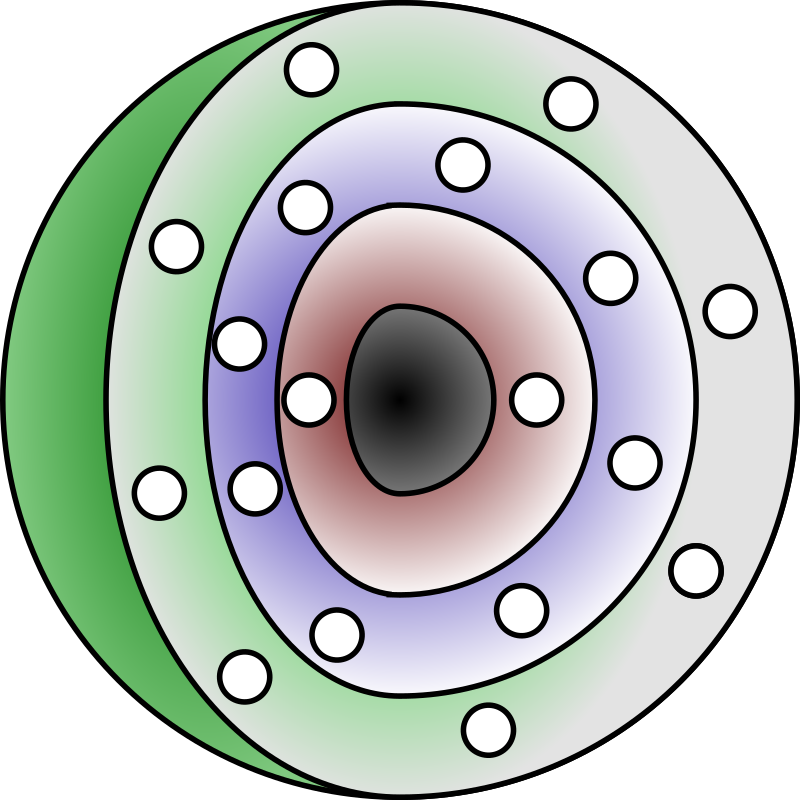
Each electron shell can only hold a certain number of electrons.
The innermost shell is the smallest and is filled with electrons first, followed by the outer shells.
The shells are numbered 1, 2, 3 etcetera. These numbers correspond to the principal quantum numbers (n = 1, 2, 3, 4 …)
Shells can also be labeled alphabetically with the letters K, L, M, …
What are orbitals? (advanced)
The concept of orbitals comes from quantum mechanics, a later model of the atom which replace Bohr’s simple model. Schrödinger was able to calculate the energy levels of hydrogen by treating a hydrogen atom’s electron as a wave, represented by the wave function Ψ.
The solutions to Schrödinger’s equation are distributions of probabilities for electron positions and locations, called orbitals. These can be imagined as “clouds” of possible locations in which an electron might be found, a distribution of probabilities rather than a precise location
Orbitals have a range of different shapes in three dimensions.

What is the difference between shells and orbitals?
Shells and orbitals are similar but different.
In the quantum mechanical model, each shell consists of one or more subshells, and each subshell consists of one or more atomic orbitals.

Electron shells of the first 20 elements
In the first twenty elements, the third electron shell is temporarily full when it holds 8 electrons.
In this course, we only need to know the shell configuration for the first 20 elements. Since 2 + 8 + 8 = 18, so we only need to consider 2 electrons in the 4th shell.
| Shell | Number of electrons it holds for the first 20 elements | Number of electrons it can hold (for all the elements) |
| 1 | 2 | 2 |
| 2 | 8 | 8 |
| 3 | 8 | 18 |
| 4 | 2 | 32 |
How do we write the electron configuration?
The electron configuration of Magnesium is 2, 8, 2
How to represent electron configuration in diagrams?
Group electrons in pairs to keep track of how many you have drawn. It also reflects the fact that electrons exist in pairs. This will also help you draw dot and cross diagrams later.
The structure of the Periodic Table
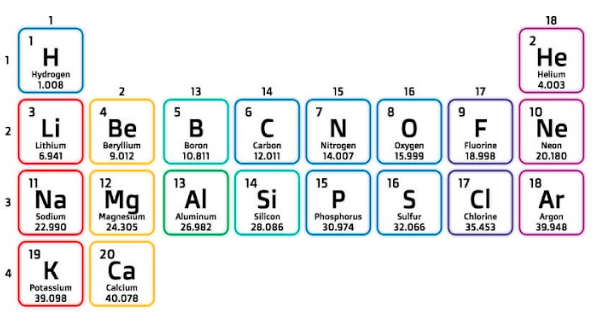
This is a table of the first 20 elements.
The vertical columns of the periodic table are called groups. We will talk about how we number the groups later.
The horizontal rows of the periodic table are called periods. They are simply numbered 1, 2, 3 etc.
Why does the periodic table have its structure?
It is important to remember that when the periodic table was first developed, nothing was known about electrons.
Chemists at the time constructed it based on the known physical and chemical properties of the elements.
However, since we’ve been learning about atomic theory, you can now see the deep, underlying structure.
What is the structure of the periodic table?
The group number represents the number of electrons in the outer electron shell.
What is special about the first 20 elements?
The electron shells fill in order for the first 20 elements.
The filling of electron shells becomes more complex in larger atoms, so you are not expected to know the electron configuration for atoms with atomic numbers greater than 20.
How is the periodic table organised?
The modern periodic table is ordered by atomic number.
Early chemists organised the periodic table by atomic weight, because the atomic number of the elements was unknown. The order is almost the same as the atomic number, but not quite. Can you find the elements which are not ordered by atomic weight?
Physical trends in the periodic table
What are the trends in atomic radius?

Why is there a trend in atomic radius?
The radius of an atom is governed by two factors:
- The number of layers of electrons (shells) around the nucleus
- The force exerted on the electrons by the nucleus
As you move across a period from left to right, the number of protons in the nucleus increases but the outer electrons are in the same electron shell. The electrostatic force on the electrons increases so the outer shell is drawn closer to the nucleus.
Valency
What is valency?
The valency of an element can be defined as the ‘combining power’ of the atoms with other atoms when it forms chemical compounds or molecules
Valency can be defined as the number of H atoms that can combine with the element.
“Valency is a measure of the number of bonds that an atom can form” (TASC 2022)
Why is there a trend in valency?
Atomic bonding (and chemical reactions) happens so that atoms get a full outer electron shell.
The electrons in the outer electron shell are called the valence electrons.
The valency of an element is equal to the number of electrons that each atom needs to gain, lose or share to fill its outer shell.
For example, the chlorine atom has only seven electrons in its outer shell, which can hold eight electrons. By gaining one electron, its outer shell becomes full. Chlorine therefore has a valency of one.
Ions
What are ions?
When atoms gain or lose electrons they are called ions.
Atoms gain or lose electrons to become more stable.
Because electrons have a negative charge, when atoms gain electrons, they become negative charged.
Conversely, when atoms gain electrons, they become positively charged.
The more electrons lost or gained, the more charged the ion.
How do we name ions?
Positively charged ions are called cations. Negatively charged ions are called anions.
Positive ions keep the name of the atom from which they are made. So, a positive ion made from a copper atom is called a copper ion.
Negative ions, on the other hand, have names that are slightly different from those of the atoms from which they are derived.
In general, the name of a negative ion is found by using the first part of the atom’s name and then adding ‘ide’ to the end of it.
For example, the negative ion formed by fluorine is called a fluoride ion.

