Why do atoms form chemical bonds?
Chemical bonding occurs as atoms combine to achieve more stable structures, i.e to gain a complete outer electron shell (or valence shell).
“Chemical bonds are caused by electrostatic attractions that arise because of the sharing or transfer of electrons between participating atoms” (TASC)
“The valency is a measure of the number of bonds that an atom can form” (TASC)
What are electrostatic forces?
The electrostatic force exists between electric charges which are at rest (stationary; static means not moving).
The magnitude of the force depends on the magnitude of the charges and the distance between them.
It’s called the electrostatic force, to distinguish it from forces experienced by electric charges that are moving. When charged particles are moving in a magnetic field, they can experience a magnetic force.
What are the types of chemical bonding?
Three types of bonding are possible:
- Metallic bonding: metallic atoms only combine to form a metallic lattice.
- Ionic Bonding: metallic atoms donate outer electrons to non-metallic atoms, to form an ionic lattice.
- Covalent Bonding: non-metallic atoms share outer electrons to form either molecules or covalent lattices.
Metallic bonding
What are the physical properties of metals?
Our model of the structure of metals aims to explain the following physical properties:
- Lustre: metals have a shiny surface when polished
- Melting Point: metals have a range of melting points but most are quite high
- Heat conduction: they are good conductors of heat
- Electrical Conduction: they are good conductors of electricity
- Malleability: metals can be hammered, bent or rolled into any desired shape
- Ductility: metals can be drawn out into wires
- Density: metals have high densities
- Strength: metals are often hard and tough with high tensile strength, meaning that they offer high resistance to the stresses of being stretched or drawn out and therefore do not easily break.
What is special about the electrons in metals?
The outer electrons are loosely bound in metal atoms.
In metals, the electrons leave the outer shells of metal atoms, forming positive metal ions and a ‘sea’ of unbound electrons that are free to move between the ions.
These electrons are referred to as delocalised.
What is the structure of metals?
Metals are solids at room temperature, so the structure of a solid metal consists of closely packed metal ions (the nuclei and inner electrons of the metal atoms).
These ions are arranged in a regular way to form what is called a lattice structure. The arrangement of the ions is different for different metals.
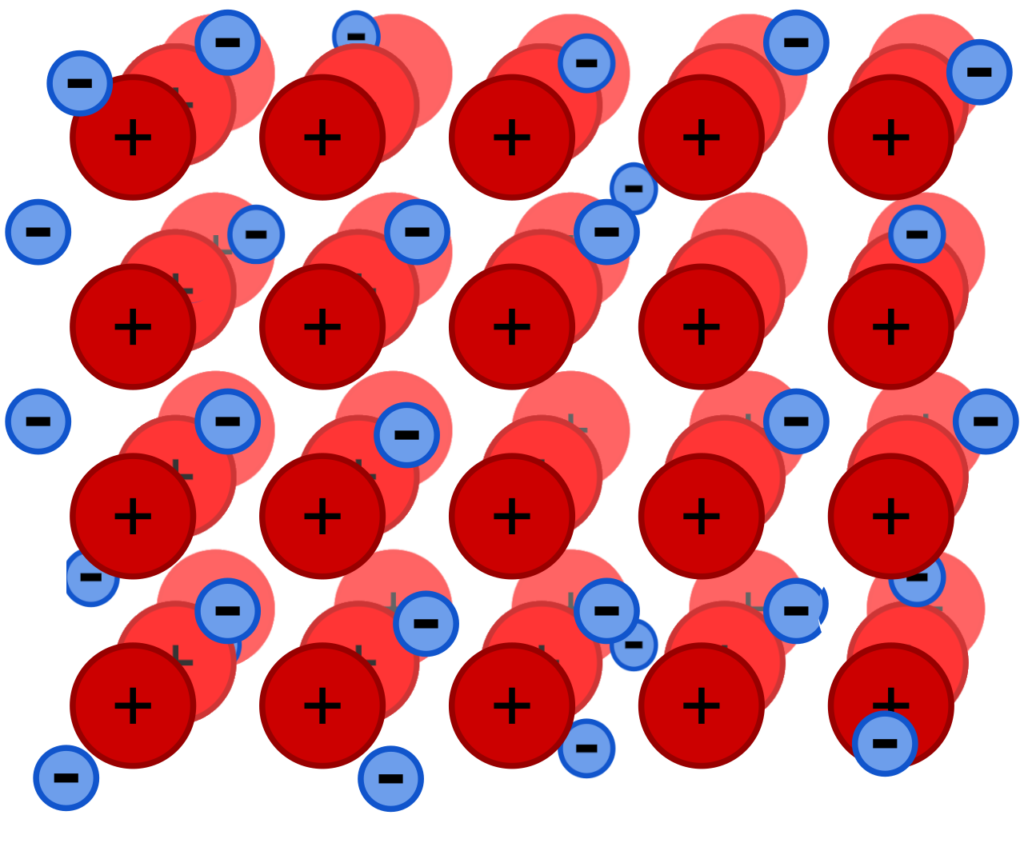
This is the reason why metals are crystalline (they have a crystal, repeating structure). The crystals are usually very small, but their obvious in some types of chemical reactions.
What is a metallic bond?
The delocalised electrons are shared by all the atoms and thus the bonding is non-directional.
The forces binding the atoms together in the lattice are the strong electrical attractions between positive ions and the sea of delocalised electrons.
What is the trend in melting point?
Generally, metals have high melting points (with at least one notable exception).
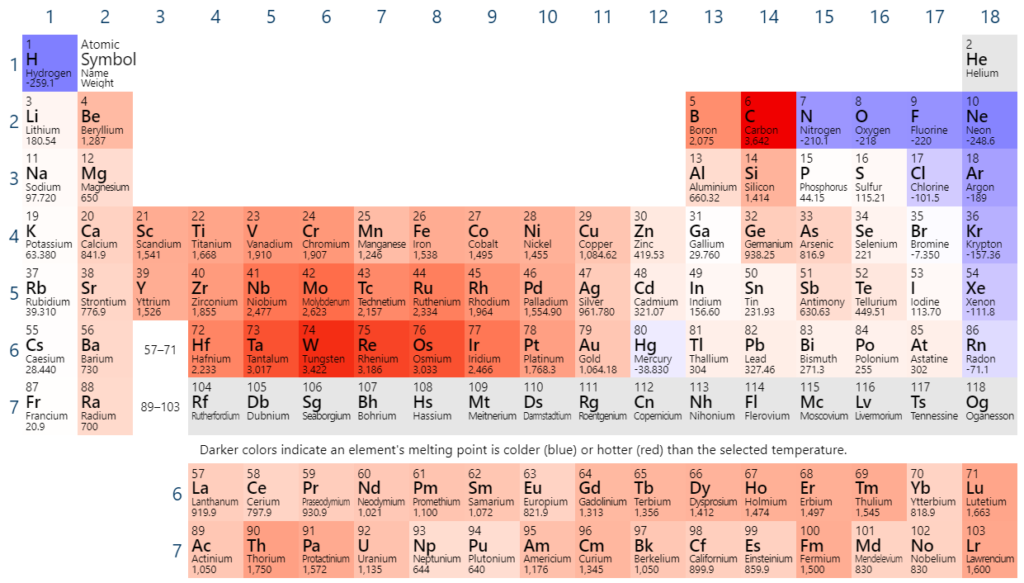
There is also a general trend of increasing melting point as you move to the right in the periodic table
What is the trend in hardness?
Metals are generally harder than non-metals, and there is a trend of increasing hardness from group 1 to 2 in all periods.
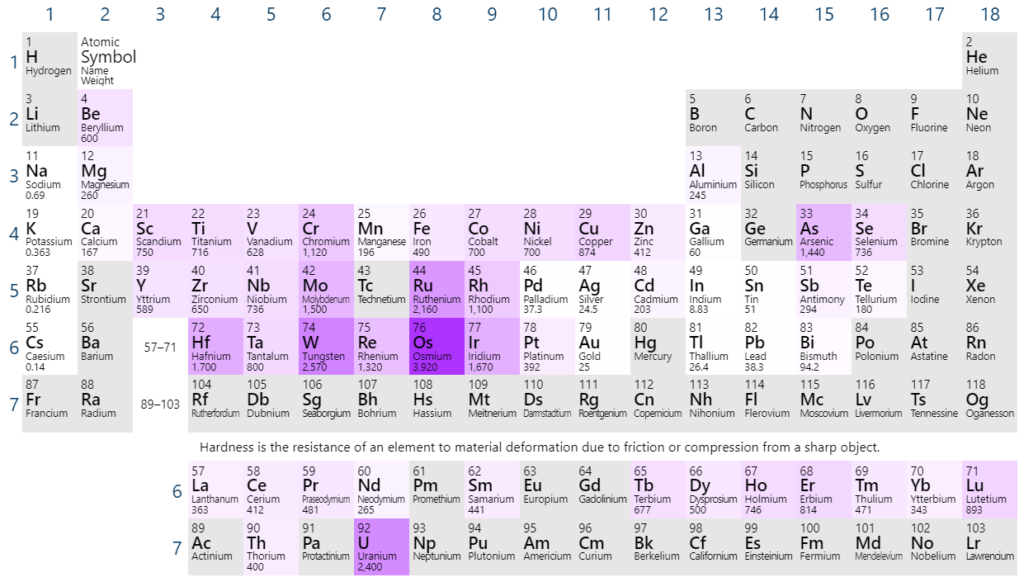
How to explain the melting point and hardness?
In sodium, only one electron per atom is involved in the metallic bond, the single outer shell electron. In magnesium, both of its outer electrons are involved, and in aluminum all three are involved.
Melting and boiling points increase across the three metals because of the increasing strength of their metallic bonds. The number of electrons which each atom can contribute to the delocalized “sea of electrons” increases. The atoms also get smaller and have more protons as you go from sodium to magnesium to aluminum. The strength of metallic bonds increases (the forces of attraction increase) because:
- The nuclei of the atoms are more positively charged.
- The “sea” is more negatively charged.
- The “sea” is progressively nearer to the nuclei and thus is more strongly attracted.
Why do stronger bonds lead to higher melting/boiling points?
When a metal melts or boils, this is a change of physical state.
Energy is transferred to a substance to melt or boil it. This energy is needed to overcome the forces of attraction between the metal ions and the delocalised electrons in the metal. The more energy needed, the higher the melting point or boiling point.
As metals are giant lattice structures, the number of electrostatic forces to be broken is extremely large, and so metals have high melting and boiling points.
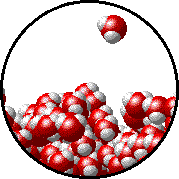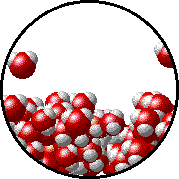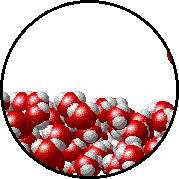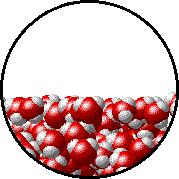
Image source: https://www.chem.purdue.edu/gchelp/liquids/tension2.html
The image above shows the interface between water in liquid and gasesous state.
How to explain the density of metals?
A substance with a high density means it has a high mass for its volume. For example, the volume of liquid in a can of drink is 330 cm3. If the can was filled with sodium (density = 0.97 g/cm3) then the can would have a mass of around 320 g. However, if the can was filled with lead (density = 11.34 g/cm3), then the can would have a mass of nearly 3.75 kg!
The close packing of the atoms in the crystal explains the relatively high density of metals compared to non-metals.
How to explain different density of metals?
Differences in the density of different metals can be partly explained by the differences in how the atoms are packed.
For example, Sodium is 8-coordinated with each sodium atom interacting with only 8 other atoms. Magnesium and aluminum are each 12-coordinated, and therefore packed more efficiently, creating less empty space in the metal structures and stronger bonding in the metal.
Sodium can float on water!
How to explain malleability and ductility?
The structure of metals consists of layers of metal ions. These layers can slide over each other when a force is applied. This means that the layers of the metal can be hammered flat, and they can also slide over each other to make thin wires.
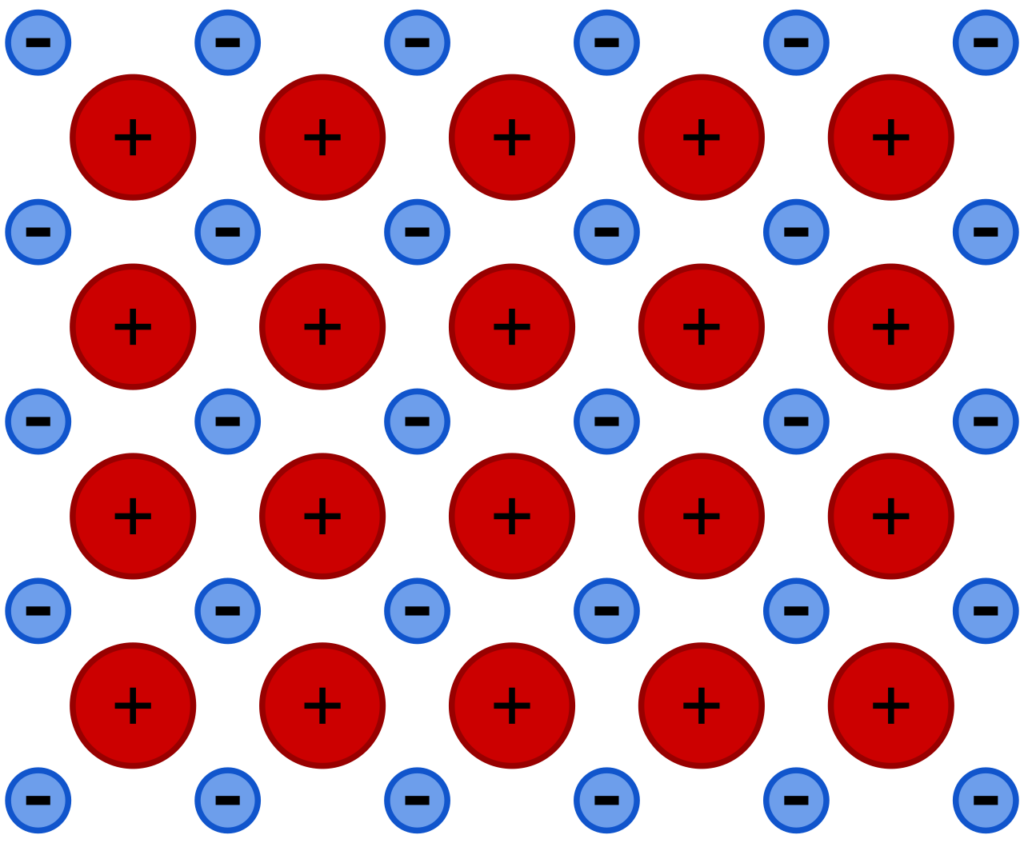
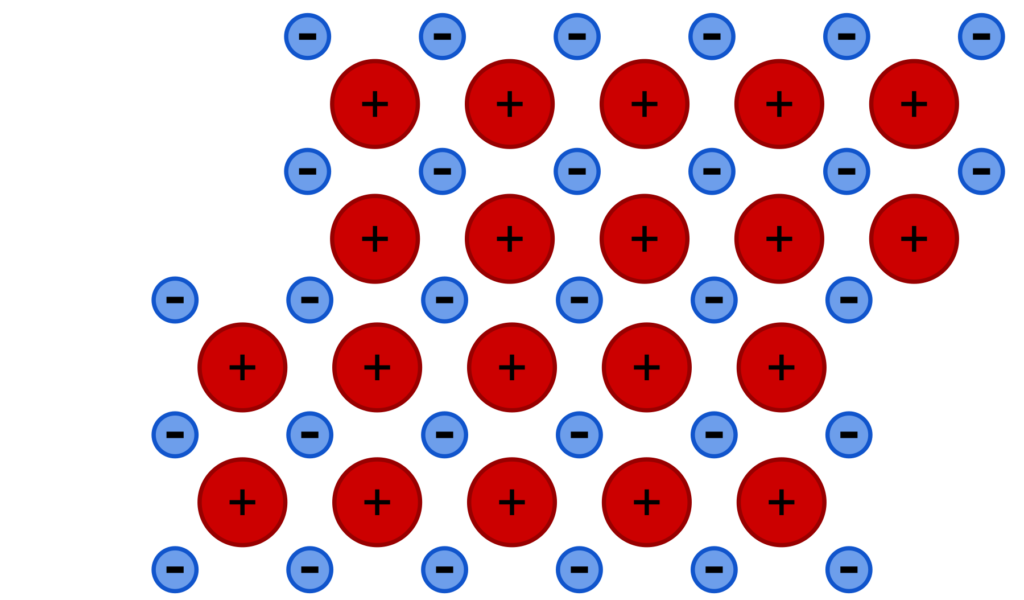
A lattice of metal ions before a force is applied (above) and after (below), which displaces some of the positive metal ions.
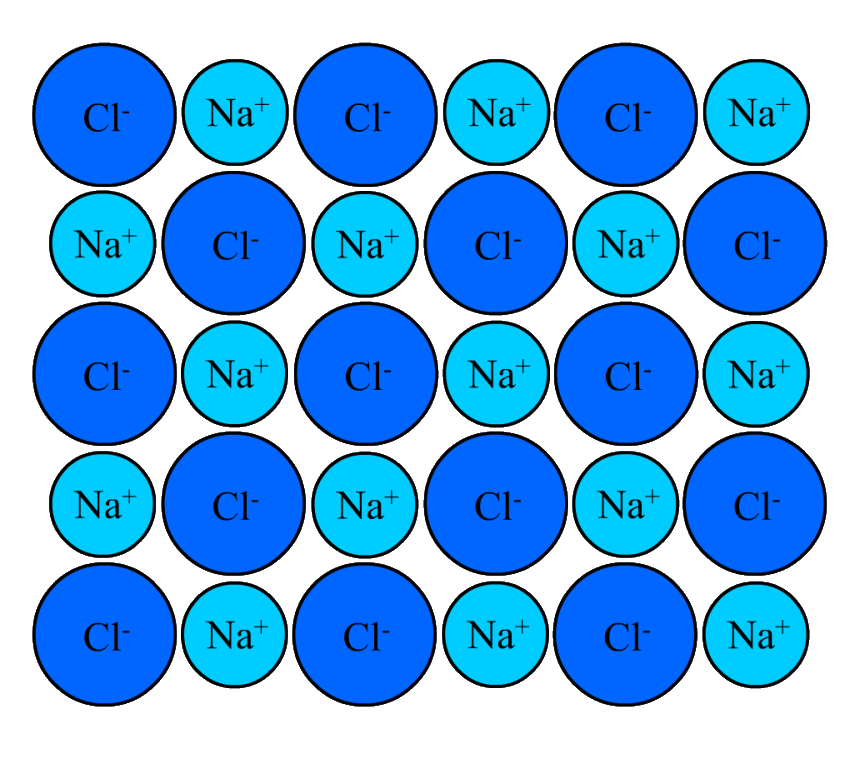
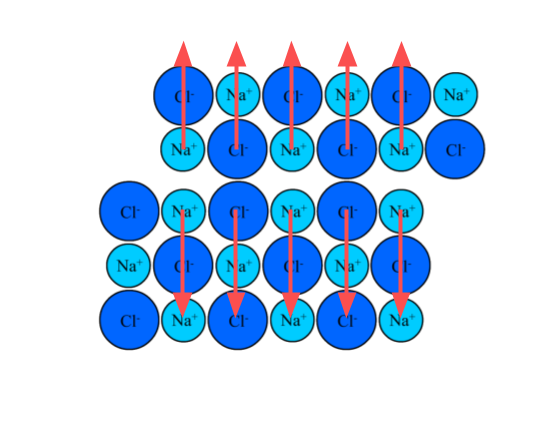
A lattice of sodium choride before a force is applied (above) and after (below), which displaces some of the ions. This results in net repulsive forces between adjacent ions with the same charge.
When the layers slide over each other, the force of attraction remains. This contrasts with ionic lattices, in which a shift in one layer can result in repulsive forces.
Metallic bonding allows the metal to change shape without shattering.
Why are metals shiny (lustrous)?
The explanation goes beyond the level of understanding required in this course but it’s pretty interesting!
How to explain electrical conductivity?
Conductivity is a measure of an element’s ability to conduct electricity or heat.
Current is the flow of electric charge. Thus, for something to conduct electricity, there must be mobile charge carriers that can flow. This can be electrons or ions.
Metals conduct electricity because the delocalized electrons (as in the “sea of electrons” model) are free to move throughout the solid or the liquid metal.
Non-metal elements do not conduct electricity because they are simple molecular substances without free, delocalized electrons. (There is at least one exception).
Why is there a trend in electrical conductivity?
Sodium, magnesium and aluminum are good conductors of electricity. Conductivity increases from sodium to magnesium to aluminum.
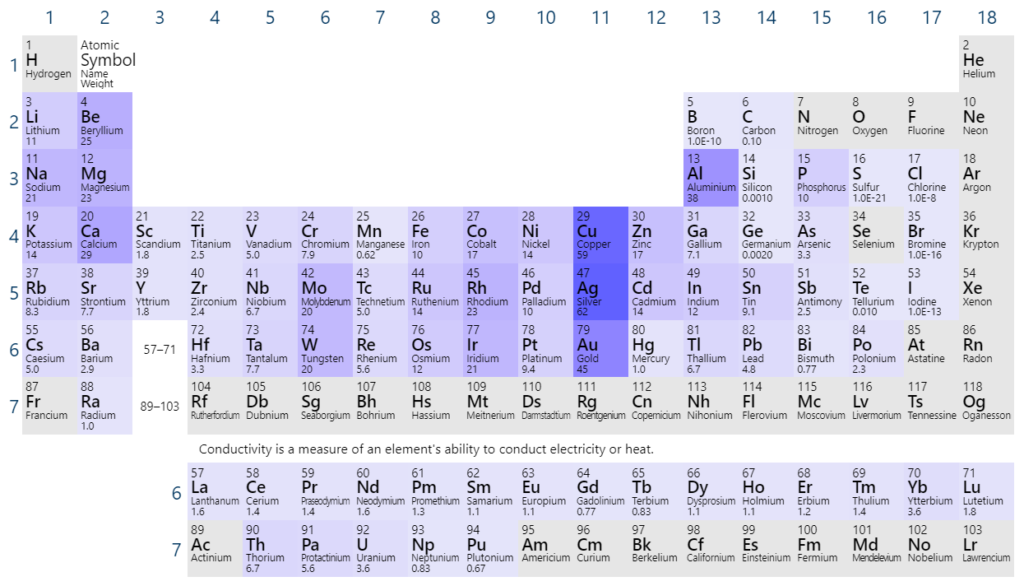
In sodium, only one electron is delocalised, but there are two delocalised electrons per magnesium atom and three per aluminium atom. The more delocalised electrons, the more charge can flow, and the better the conductor.
What is conduction of heat?
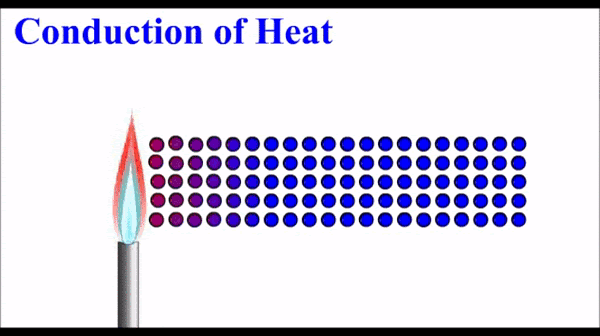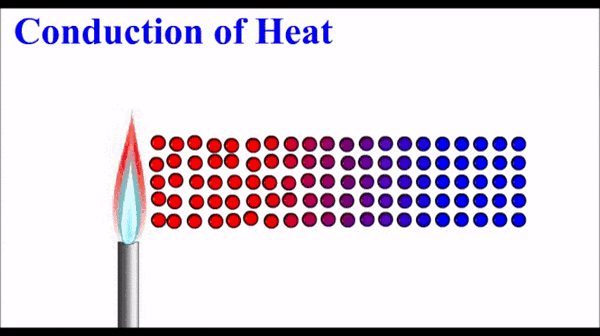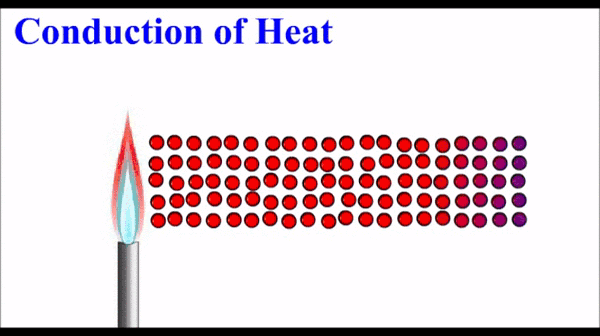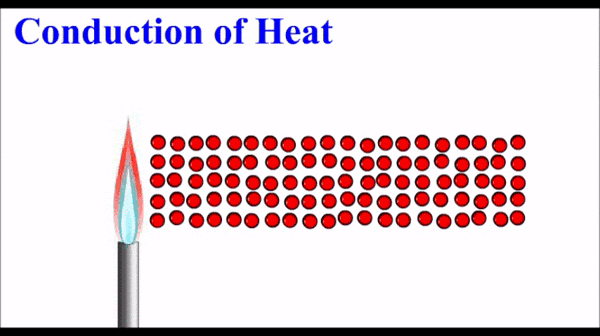
Conduction is one method of heat transfer (the transfer of thermal energy). It occurs in solids and fluids.
Metals have quite high thermal conductivity compared to non-metals. Note that the pattern in thermal conductivity is virtually identical to the pattern for electrical conductivity.
How does conduction occur in metals?
The free electrons in a metal are in thermal equilibrium with the positive ions that make up the atomic lattice of the solid. (They have the same energy).
The electrons in a metal can interact with each other and the energy from the high temperature end of the solid “diffuses” along the solid by interactions (e.g. collisions) between these electrons. When an electron interacts with a metal ion, the energy is transferred back into the atomic lattice to make the ions vibrate. This vibration is energy and is observed/measured as temperature.
Recommended summary videos on Metallic bonding
Ionic bonding
What is a molecule?
It is important to understand the concept of a molecule before discussing different types of chemical bonds.
Many substances that exist as two or more atoms connected together so strongly that they behave as a single particle. These multi-atom combinations are called molecules.
A molecule is the smallest part of a substance that has the physical and chemical properties of that substance. (In this way, a molecule is similar to an atom. If we split apart a molecule of water, it is no longer water. A molecule, however, is composed of more than one atom.)

By Booyabazooka at English Wikipedia – Transferred from en.wikipedia to Commons by Jay8g using CommonsHelper., Public Domain, https://commons.wikimedia.org/w/index.php?curid=16278333
Ions
What are ions?
Please review Ions in 4.1 Properties and structures of atoms
What are polyatomic ions?
Sometimes, whole molecules become ions. They do not separate in chemical reactions.
Examples of polyatomic ions include:
- Carbonate – CO32-
- Sulphate – SO42-
- Nitrate – NO3–
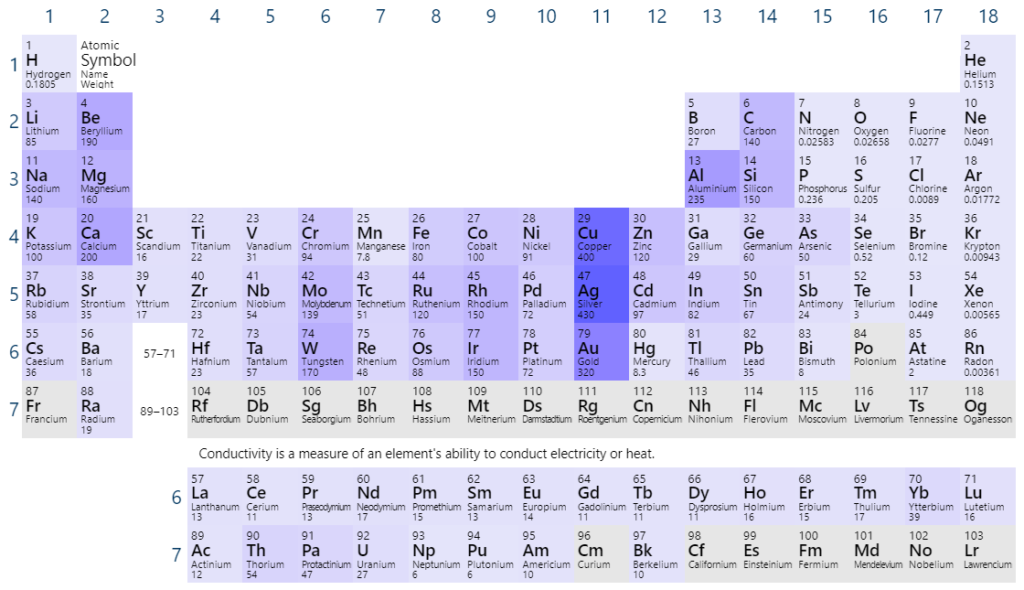
- Hydroxide – OH– (see image)
You won’t need to remember the names, formulae or charges on these ions because they’re given on your information sheet.
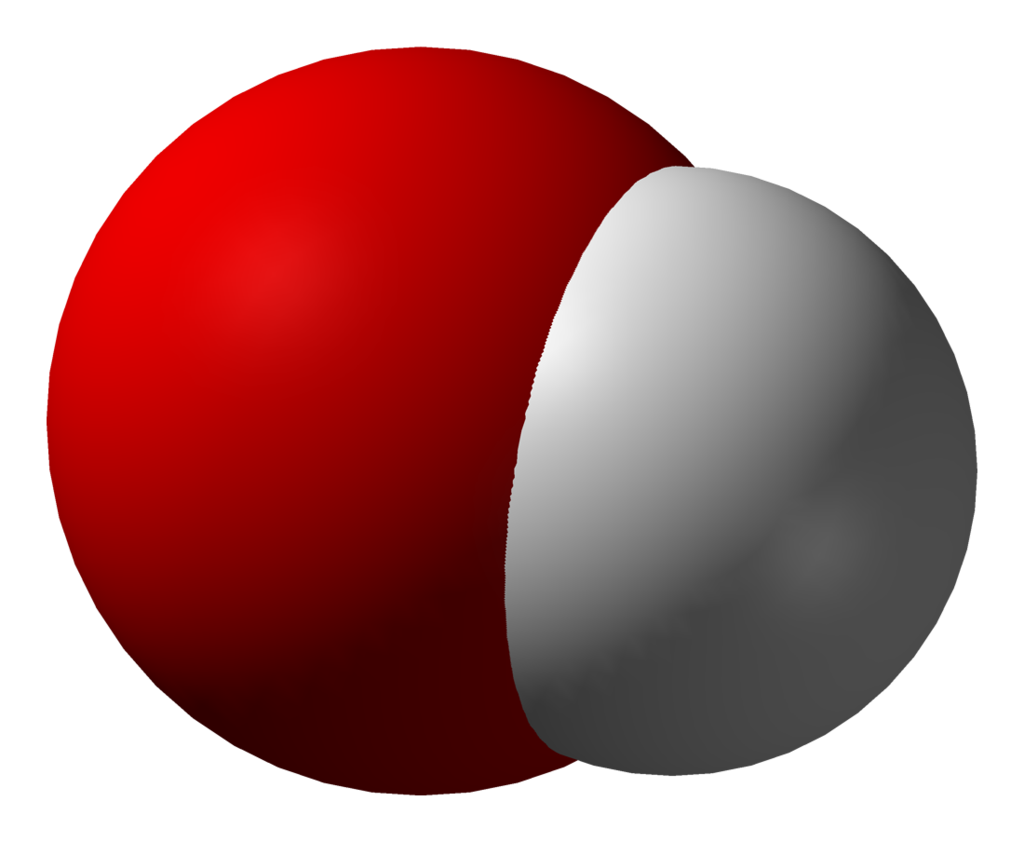
Three-dimensional model of the hydroxide ion. By Benjah-bmm27 – Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=1690806
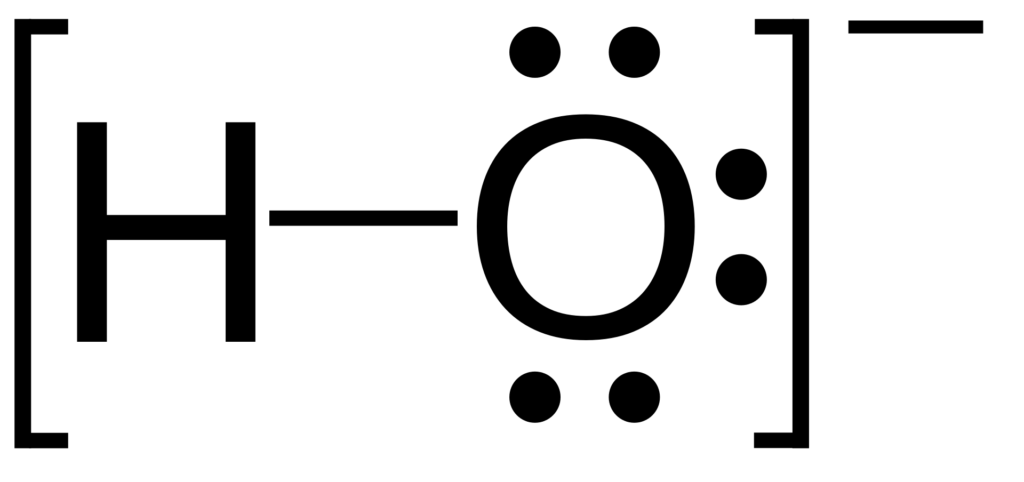
Structural formula of the hydroxide ion. By DoSiDo – Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=2803509
What are ionic compounds?
Ionic bonds always form compounds (unlike covalent bonds). A compound is a substance that is made from atoms of different elements bonded to each other.
Ionic compounds are known as salts.
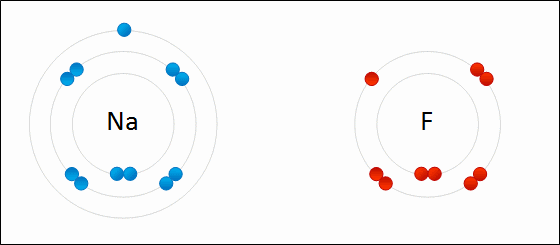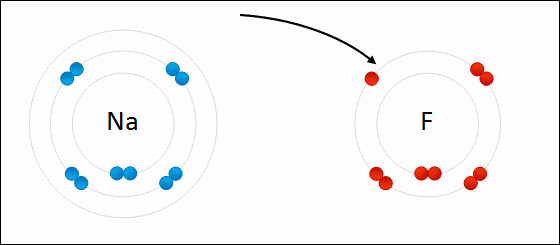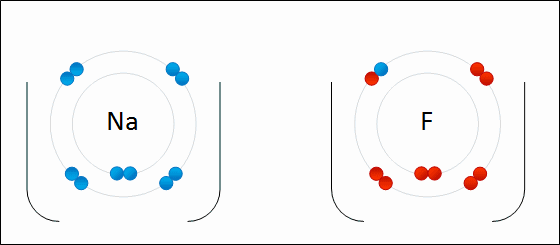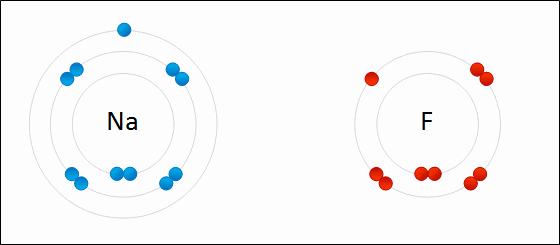
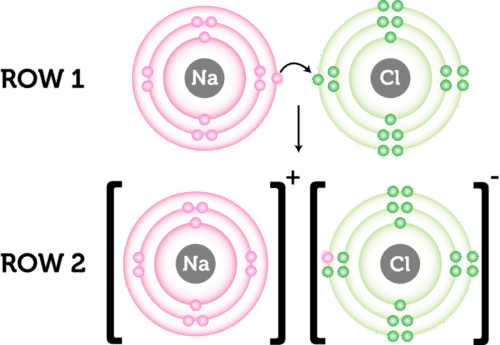
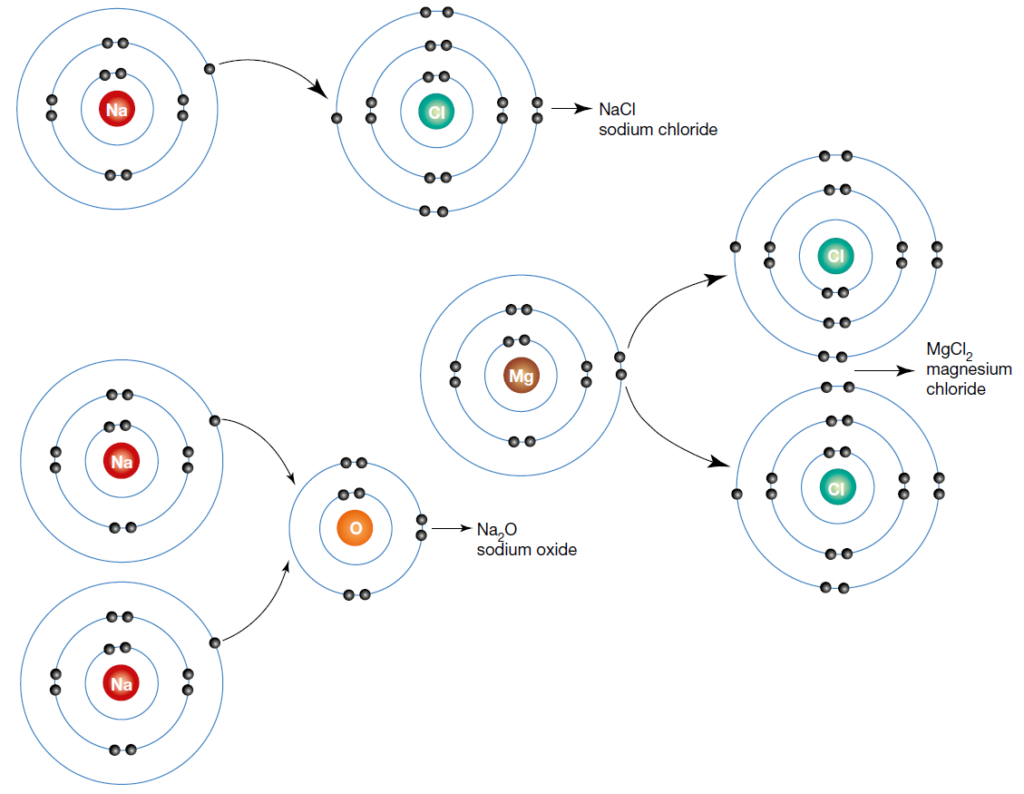
Ioic bond formation is often represented using the reaction between metal and non-metal elements (e.g. between Na and F) as shown above. This reaction involvees ion formation immediately followed by the formation of ionic compounds. One atom essentially gives up an electron to the other, forming positive and negative ions.
Note that many reactions that result in the formation of ionic bonds (e.g. precipitation reactions) occur between species that are already in ionic form.
What is an ionic bond?
The ions are held together by electrostatic forces. We call the forces that hold the ions together Ionic bonds.
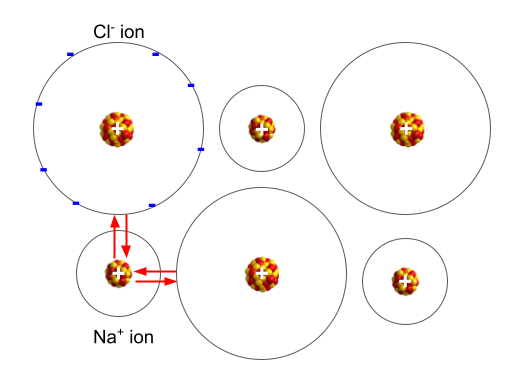
There is a force of attraction between the ions.
It’s the net force acting between the positively charged nucleus of the cation and the negatively charged electrons of the anion.
The distance between the ions and the magnitude of the charges affects the magnitude of the force
Why do ionic compounds form?
When the elements in an ionic compound form ions, they achieve a more stable state.
The elements are in a more stable state when they form ions because they have a full outer electron shell.
When ionic bonds form between only two elements, the compound formed is called a binary ionic compound.
E.g. LiF, or sodium fluoride is made of Na+ and F– ions)
What is the octet rule?
The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell.
It’s a nice shorthand way of explaining a simple concept. We can simply say that an atom follows the octet rule when it is gaining/losing/sharing electrons to gain a full outer electron shell.
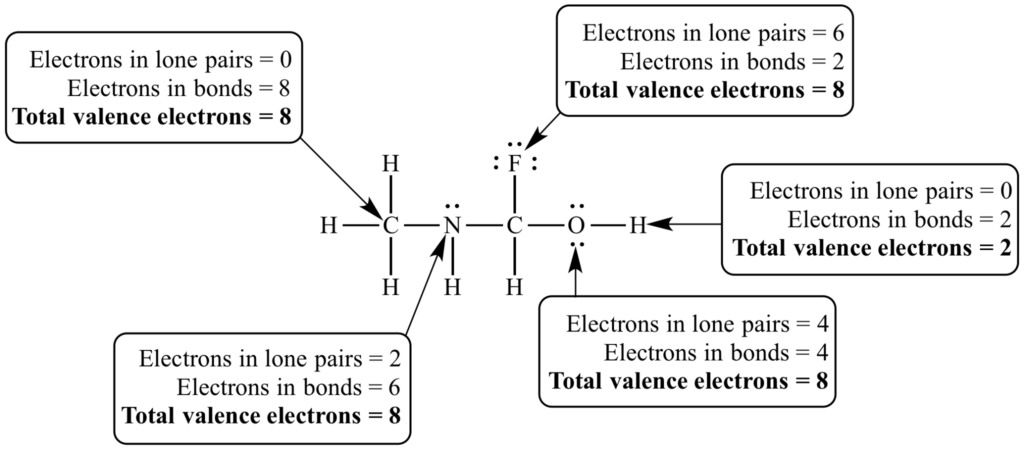
Hydrogen follows its own rule: it has a tendency to prefer to have two electrons in the valence shell. Because the first energy level of electrons in an atom holds a maximum of two valence electrons, hydrogen only needs one more electron to complete its valence shell. This sometimes called the duet rule.
How to represent ionic bond formation?
It’s common to:
- Show the process in steps
- use arrows to show the transfer of electrons
- Use brackets around ions to indicate that they are charged
- Give the charge on the ions
What is the structure of ionic compounds?
Ionic compounds form lattices, not molecules.
A lattice is a regular, repeated three-dimensional arrangement of atoms or ions.
An ionic lattice is an ordered structure of ions held together by electrostatic attraction between the positive and negatively charged ions.
Ionic compounds form crystals in their solid form because of their lattice structure.
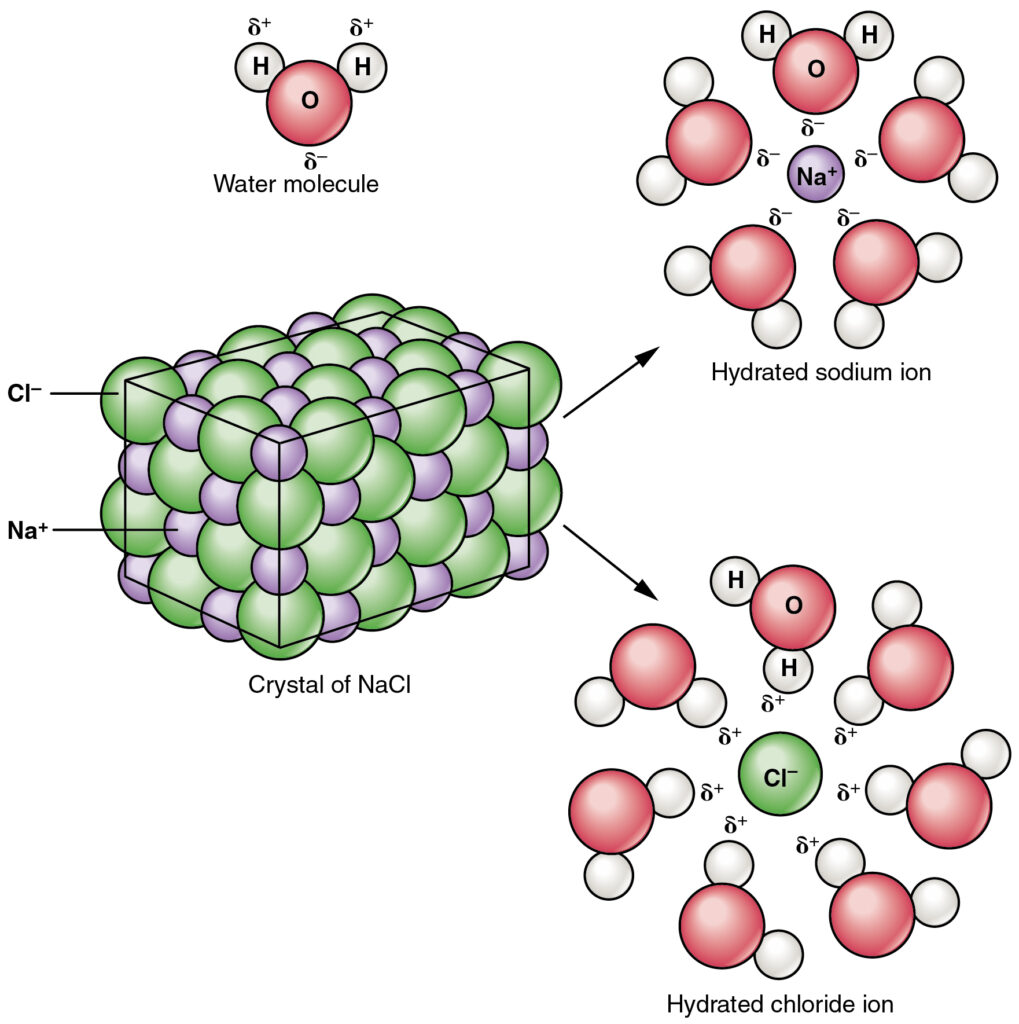
What happens when ionic compounds dissolve?
- Not all ionic compounds are soluble (can dissolve).
- If and when ionic compounds dissolve, they form positive and negative ions.
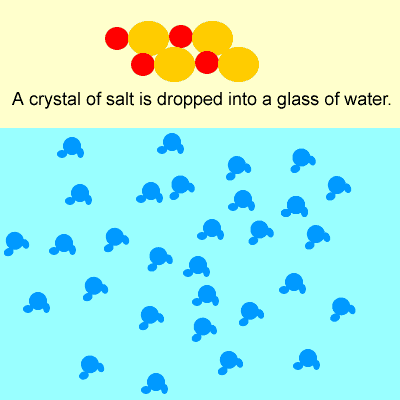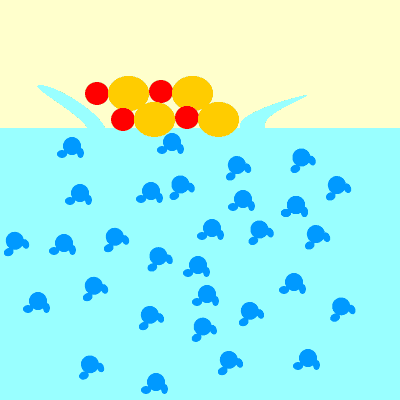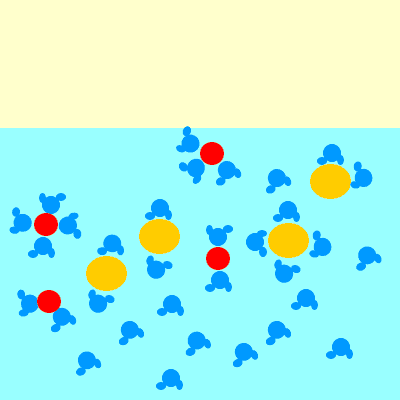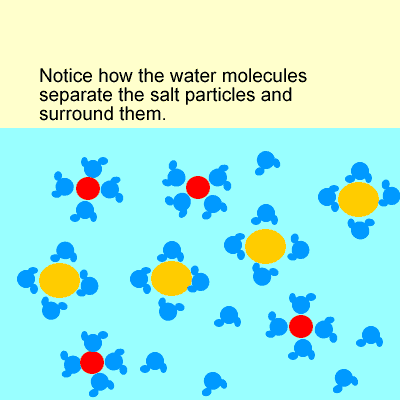
For example, when sodium chloride (NaCl) dissolves, it forms Na+ and Cl– ions.
What are the properties of ionic compounds?
Compared to covalent compounds, ionic compounds:
- have high melting points,
- are brittle,
- are able to conduct electricity when molten or in solution
How to explain these properties?
Why are ionic compounds brittle?
Ionic compounds are generally hard, but brittle. Why? It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. However, when that happens, it brings ions of the same charge next to each other.
The repulsive forces between like-charged ions cause the crystal to shatter.
When an ionic crystal breaks, it tends to do so along smooth planes because of the regular arrangement of the ions.


Why do ionic compounds have high melting points?
The strength of any bond depends upon the charges of the particles involved and the distances between them. Because ionic bonds involve the transfer of electrons, the ions have larger charges and there is more force between them.
Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong.
The process of melting an ionic compound requires the addition of large amounts of energy in order to break all of the ionic bonds in the crystal. For example, sodium chloride has a melting temperature of about 800°C.
Why do ionic compounds conduct electricity?
Current is the flow of electric charge. Thus, for something to conduct electricity, there must be mobile charge carriers that can flow. This can be electrons or ions.
Molten or dissolved ionic compounds can conduct electricity because the ions are mobile and carry electric charge.
Formulae of chemical compounds revision
What is the difference between formulae and symbols?
These are symbols: H (hydrogen), C (carbon)
These are formulae*: O2 (oxygen gas – a molecule), CO2 (water – a molecule).
The numbers after the element symbols represent how many atoms there are of each element in the compound. E.g. CH4 made of one Carbon atom for every four Hydrogen atoms. We never write the one (e.g. never C1H4).
If there are brackets, the number after the brackets multiplies everything inside the brackets. E.g. in Mg(NO3)2 there are two nitrate (NO3–) ions for every magnesium Mg+ ion.
*formulae is the plural of formula
Ionic compounds in detail
What is the formula of an ionic compound?
An ionic compound can be described using a chemical formula, like NaCl.
NaCl is an empirical* formula. This formula merely indicates that sodium chloride is made of an equal number of sodium and chloride ions.
NaCl is NOT made of molecules (groups of atoms).
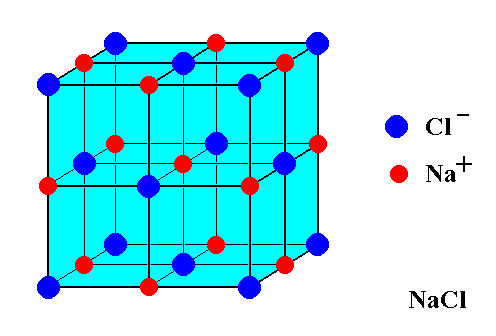
The chemical formula describes the ratio of the cations and anions in the compound e.g. in NaCl there is one sodium ion for every chloride ion.
*explained in next topic
How to name ionic compounds?
- The cation is written first in the name; the anion is written second in the name.
- Remember from 4.1:
The name of the cation is the same as the (neutral) element from which it is derived (e.g., Na+ = “sodium”).
The anion is named by adding the suffix -ide to the root of the element name (e.g., I– = “iodide”).
E.g. sodium iodide, NaI, is made of sodium ions Na+ (metal ions), and iodide ions, I– (non-metal ions).
Note: For ionic compounds, the greek prefixes (mo, di, tri etc.) are not used to indicate the number of atoms of each element in the formula unit for the compound. (e.g., Na2O is named “sodium oxide” not “disodium oxide”, or “disodium monoxide”). This is different to covalent molecules.
What is the overall charge of an ionic compound?
The ions of an ionic compound combine so that the total or overall charge is zero.
In NaCl, for every Na+ ion with a charge of 1+, there is a Cl– ion with a charge of 1-. The total charge is (1+) + (1-) = 0
In CaCl, for every Ca2+ ion with a charge of 2+, there are two Cl– ions, each with a charge of 1-. The total charge is (2+) + [2 ⨯(1-)] = 0
In simplified scenarios involving ion formation followed by the formation of ionic compounds (see image), all the electrons lost by positive ions will be gained by negative ions.
How to work out the formula of an ionic compound?
The very first step is to use a periodic table to find out the ions formed by the elements in the compound.
In this course, you can use the tables of common positive ions (cations and anions) found on page 4 of your information sheet.
This video explains how the ions combine to make a neutral compound in which the charges balance.
What ions are formed by the transition metals?
Some transition metals have complicated electron arrangements and can form ions that have different charges.
These ions have different physical and chemical properties and form different binary ionic compounds with different physical and chemical properties.
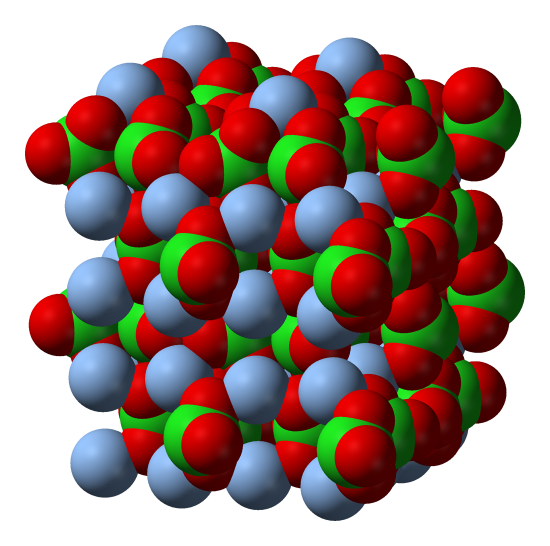
E.g. Copper can form Cu1+ or Cu2+ ions. CuCl2 (containing Cu2+), shown above, is brown, while CuCl (containing Cu1+) shown below is white.


How to distinguish between different ions?
Roman numerals in brackets after the cation’s name are used to denote its charge.
For example: Iron can form black iron (II) oxide and brown iron (III) oxide.
The black iron (II) oxide contains Fe2+ ions. The brown iron (III) oxide contains Fe3+ ions.
Practice later: What is the chemical formula of each of these compounds?


How do polyatomic ions form compounds?
The atoms in a polyatomic ion are usually covalently bonded to one another, and therefore stay together as a single, charged unit.
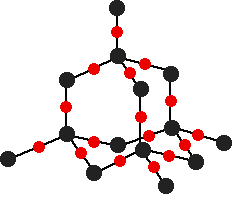
The diagram shows the lattice structure of Silver chlorate, AgClO3. The silver spheres representing Ag+ and the red and green molecules representing the polyatomic chlorate ion, ClO3–.
The overall charge of Silver chlorate is zero. There is one silver ion for every chlorate ion.
How to use the “cross method” or “swap and drop”?
Start by writing the symbols of the ions including their charges, putting the cation first
Write the ion symbols close to each other, leaving a small gap between the symbols
Swap and drop: Write the number of the charge of one ion as a subscript after the other ion. Do not write the number if it’s a one.
Write the formula without gaps if needed.
How to write formulae with polyatomic ions?
The positive ion (cation) is written first in the name, even when that is a polyatomic ion like ammonium (NH3+); the negative ion (anion) is written second in the name.
When the formula contains two or more of the same polyatomic ion, that ion is written within parentheses and a subscript is written outside the parentheses to indicate the number of polyatomic ions.
Exception: parentheses and a subscript are not used unless more than one of a polyatomic ion is present in the formula unit (e.g., calcium sulfate = “CaSO4” not “Ca(SO4)”; ammonium carbonate = “(NH4)2CO3” not “(NH4)2(CO3)”).
The rules for binary ionic compounds also apply.
Precipitation reactions
What are precipitation reactions?
When ionic compounds dissolve, they form positive and negative ions in solution.
When two different ionic compounds in solution are mixed, they can react to create new substances.
If the ions can rearrange to form an insoluble ionic compound, they will.
The insoluble ionic compound forms a solid powder mixed with the water which is called a precipitate.
What happens when ionic compounds dissolve?
When ionic compounds dissolve, the ions physically separate from each other. We can use a chemical equation to represent this process—for example, with NaCl:
When NaCl dissolves in water, the ions separate and go their own way in solution; the ions are now written with their respective charges, and the (aq) phase label emphasizes that they are dissolved. This process is called dissociation; we say that the ions dissociate.
How do more complex salts dissolve?
When the ions separate, all the ions separate. Thus, when CaCl2 dissolves, the one Ca2+ ion and the two Cl− ions separate from each other:
That is, the two chloride ions go off on their own. They do not remain as Cl2 (that would be elemental chlorine; these are chloride ions); they do not stick together to make Cl2− or Cl22−. They become dissociated ions in their own right.
Polyatomic ions also retain their overall identity when they are dissolved.
What is the molecular equation for a precipitation reaction?
The image shows the reaction between sodium iodide solution and a lead nitrate solution to produce solid lead iodide (the yellow precipitate). The sodium and nitrate ions remain in solution.

sodium iodide + lead nitrate → lead iodide + sodium nitrate
A chemical equation is much more accurate:
NaI(aq) + Pb(NO3)2(aq) → PbI2(s) + NaNO3(aq)
This type of equation is called a molecular equation because the formulas of the compounds are written as though all the substances existed as molecules or whole units (you know that they don’t).
What is happening in precipitation reactions?
What is the complete ionic equation?
When chemicals in solution react, the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms of the dissociated ions, not the complete ionic formula. A complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions.
For example, when NaCl(aq) reacts with AgNO3(aq) in a double-replacement reaction to precipitate AgCl(s) and form NaNO3(aq), the complete ionic equation includes NaCl, AgNO3, and NaNO3 written as separated ions:
What are spectator ions?
the Ag+(aq) and Cl−(aq) ions become AgCl(s), but the Na+(aq) ions and the NO3−(aq) ions stay as Na+(aq) ions and NO3−(aq) ions. These two ions are examples of spectator ions: ions that do nothing in the overall course of a chemical reaction. They are present, but they do not participate in the overall chemistry.
What is the net ionic equation?
It is common to cancel spectator ions on the opposite sides of a chemical equation:
What remains when the spectator ions are removed is called the net ionic equation, which represents the actual chemical change occurring between the ionic compounds:
It is important to reiterate that the spectator ions are still present in solution, but they don’t experience any net chemical change, so they are not written in a net ionic equation.
How to predict solubility?
Many experiments have allowed us to develop a set of “solubility rules” that can be used to determine whether a compound is soluble or insoluble.
The solubility rules are provided on the information sheet for Physical Sciences students.
Using the solubility rules, it is possible to predict whether a reaction will occur.
Remember, if the ions can rearrange to form an insoluble ionic compound, they will, and a reaction will occur (a new substance will form).
How to use the solubility rules? An example.
Will a reaction occur between a potassium chloride solution and a silver nitrate solution?
The ions could re-arrange and combine to form silver chloride and potassium nitrate. Because silver chloride (AgCl) is insoluble, we know that a reaction will occur.
Thus, potassium chloride and silver nitrate will react to form a silver chloride precipitate and leave behind a potassium nitrate solution.
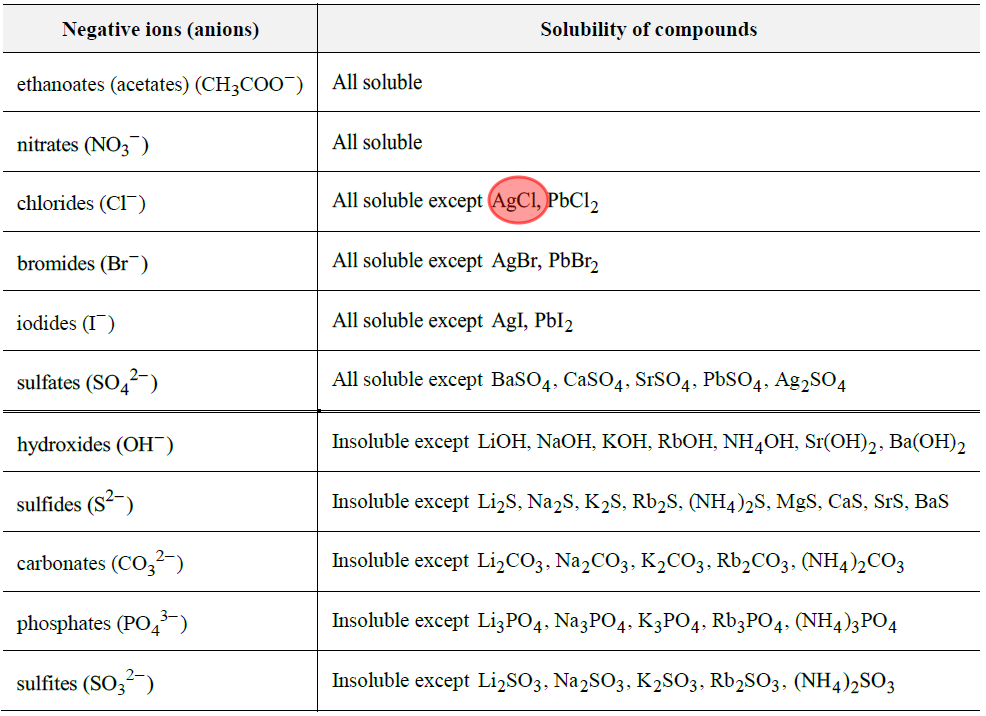
How to determine if an ion is present in solution?
It’s worth remembering that all salts of the alkali metals (Group I), plus NH4+, are usually soluble. This includes Na+, Li+, Na+, K+, Rb+, Cs+. This information is not on your solubility table.
Identifying unknown inorganic compounds based on solubility
We can use our knowledge of solubility and precipitation reactions to devise tests to identify unknown compounds.
How can precipitation be used to remove ions?
Solids are easier to separate from a mixture than dissolved substances. For this reason, precipitation reactions are often used to remove toxic dissolved substances.
Electron dot diagrams
What are electron dot diagrams?
The formation of many common compounds can be visualized with the use of electron dot diagrams (also known as Lewis symbols and Lewis diagrams).
In an electron dot diagram, the inner closed shells of electrons can be considered as included in chemical symbol for the element, and the outer shell or valence electrons are represented by dots.
Electron dot diagrams are not the same as diagrams showing the full electron configuration.
How to draw electron dot diagrams?
The reasons are beyond the scope of this course, but the pairing of electrons is important, and you must be able to draw the correct electron dot diagram for an element.
Which one of the following electron dot diagrams for oxygen is correct?
Covalent compounds
What are covalent bonds?
Molecules (groups of atoms bonded together) are made of covalent bonds.
Covalent bonds form between the atoms of non-metal elements.

In covalent molecules, valence electrons are shared so that the atoms get full outer electron shells, which is a more stable state for the atoms involved.
Why is there a force of attraction?
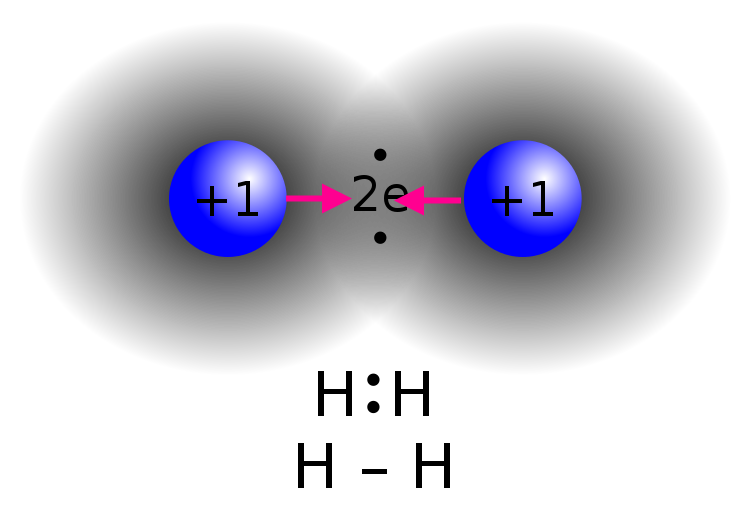
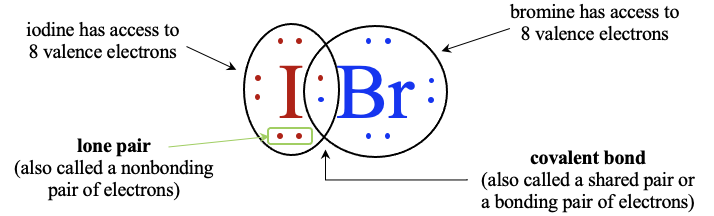
What are lone pair electrons?
Through sharing, the iodine atom now has access to eight valence electrons, as does the bromine atom. The portion where the circles overlap represent a shared pair of electrons, otherwise known as a covalent bond. Electrons that are not a part of a covalent bond are called lone pairs.
The presence of lone pairs on the central atom(s) of a molecule contributes to its three-dimensional shape.
Why do multiple covalent bonds form?
Some molecules are unable to achieve a full outer electron shell by making single covalent bonds between atoms so they make double or triple bonds.
For example, consider a molecule of oxygen, O2. Each oxygen atom has 6 valence electrons and needs two more electrons to complete its valence shell:
If the two oxygen atoms were to form a single bond with each other, they each have access to only seven valence electrons.
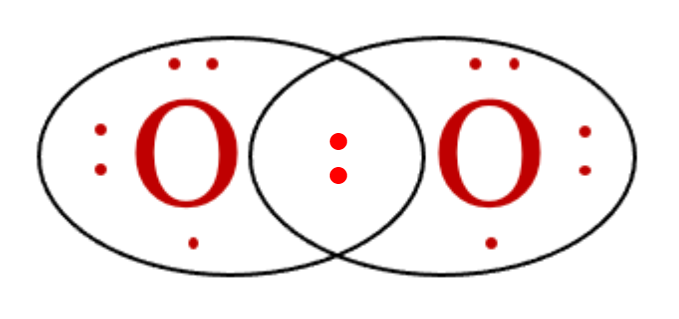
Each oxygen atom must achieve the octet rule by forming a double covalent bond, also called a double bond.

A double bond is formed when two pairs of valence electrons are shared between the same two atoms.
Similarly, A triple bond is formed when three pairs of valence electrons are shared between the same two atoms.
For example, Nitrogen:

Double and Triple bonds are stronger than Single bonds.
How to draw electron dot diagrams for molecules?
Electron dot diagrams can be used to represent covalent bonding. In electron dot diagrams, shared electron pairs are shown as pairs of dots between the two atoms and lone pairs are shown as pairs of dots on individual atoms.
Electron dot diagrams are commonly called Lewis structures/diagrams.
Can lines be used to represent bonds?
Since Gilbert Lewis first suggested using dots to represent shared electrons in diagrams of chemical compounds, the idea has been extended and simplified.
Sometimes, lines between atoms are used to represent shared pairs in a chemical bond, as shown.
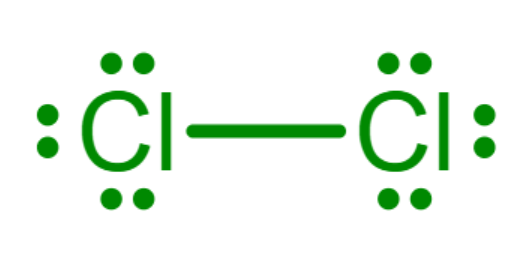
In this course, we recommend using dots, as per Lewis’ original article, because they show a greater understanding of what a covalent bond is.
The diagrams you will be required to draw will be molecules of elements and covalent compounds (e.g. simple hydrocarbons and simple common molecular compounds)
How many covalent bonds can an element form?
The normal number of covalent bonds for atoms in compounds is equal to the number of electrons required by an atom to complete its valence shell, as shown in the Table below.
Recall that only nonmetals and/or metalloids typically form covalent bonds with each other.
In this course, we defined valency as “a measure of the number of bonds that an atom can form”. The normal number of covalent bonds for an atom in a compound is equal to the valency of the atom.
When atoms form the normal number of covalent bonds with other elements, they may do so in any manner that sums to equal the normal number of bonds. In other words, they can form combinations of single, double or triple bonds.
| H | Group VI | Group V | Group VI | Group VII | |
| Number of valence electrons | 1 | 4 | 5 | 6 | 7 |
| Normal number of covalent bonds (the valency) | 1 | 4 | 3 | 2 | 1 |
Example: what bonds can carbon form?
For example, carbon (C) is located in Group IVA. This means it has four valence electrons and normally makes four covalent bonds. As a result, when carbon bonds with other elements, all of the following bond combinations are possible:
- 4 single bonds
- 2 single bonds and 1 double bond
- 1 single bond and 1 triple bond
- 2 double bonds
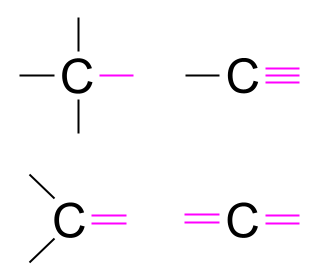
How to draw Lewis dot structures for molecules?
- Draw the electron dot diagram for the central atom. The central atom(s) will usually be the element that makes the greatest number of bonds and is surrounded by atoms that make a lesser number of bonds.
- Arrange the atoms around the central atom.
- Add electrons as pairs to make an octet around each atom in the molecule, starting with the surrounding atoms and working inward to the central atom(s).
- The octet rule is followed by every atom in the molecule. Remember that hydrogen follows the duet rule. Its valence shell holds up to two electrons.
- It’s best practice to use a different symbol for the electrons of the surrounding atoms.
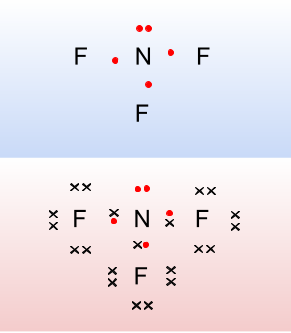
Naming and writing formulae for covalent compounds
How to name covalent molecular compounds?
- The name of the first atom in the formula is given first.
- The suffix -ide is then added to the stem of the name of the second atom.
- The number of atoms of an element in simple covalent compounds is indicated by adding one of the Greek prefixes to the name of the element.
- mono-
- di-
- Tri-
Some simple molecules have common names that we use as part of the formal system of chemical nomenclature. For example, H2O is given the name water, not dihydrogen monoxide. NH3 is called ammonia, while CH4 is called methane. We will occasionally see other molecules that have common names.
What prefixes do we use?
In some names, the final ‘a’ or ‘o’ of the prefix is dropped to avoid awkward pronunciation.
Thus OsO4 is osmium tetroxide rather than osmium tetraoxide
The prefix mono- is never used for the first element.
We usually only use the prefix mono- in front of oxygen compounds e.g. carbon monoxide.
The prefixes are provided on the information sheet for Physical Sciences students.
Example naming covalent compounds
This covalent compound is made of nitrogen and oxygen.
- We give the name of the first element.
- Then we change the name of the second element: the ‘stem’ or root of oxygen is ox- to which we add -ide.
- Finally we add prefixes to show the number of atoms.
Image source: Ball, Hill and Scott, 2012 Introduction to Chemistry: General, Organic, and Biological. CC BY-NC-SA 3.0 DEED, Attribution-NonCommercial-ShareAlike 3.0 Unported
Covalent Network Solids
What are Covalent Network Solids?
Covalent Network Solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(IV) oxide).
These covalent substances are not made of molecules.
These structures can be two-dimensional (like graphite) or three dimensional (like diamond and silicon dioxide).
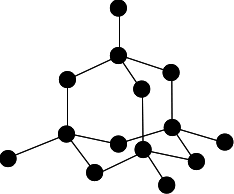
Diamond
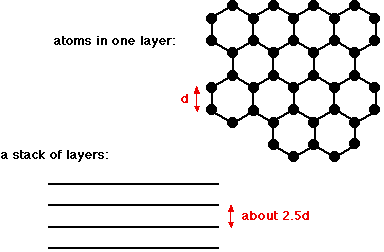
Graphite
Silicon dioxide
Images by Jim Clark, 2019 https://www.chemguide.co.uk/14to16/structure/giantcovalent.html#top
Which compounds form covalent networks?
Covalent networks often contain silicon and carbon, but predicting whether a covalent network structure will result from the bonding between two elements is beyond the level of this course.
So, you’ll just have to memorise a few of the most common:
- Diamond and graphite (C),
- Silicon (Si)
- silicon dioxide, SiO2 (quartz),
- silicon carbide (SiC)
- tungsten carbide (WC)
There are a few more, including Boron nitride (BN)
Intermolecular forces
How are intramolecular and intermolecular forces different?
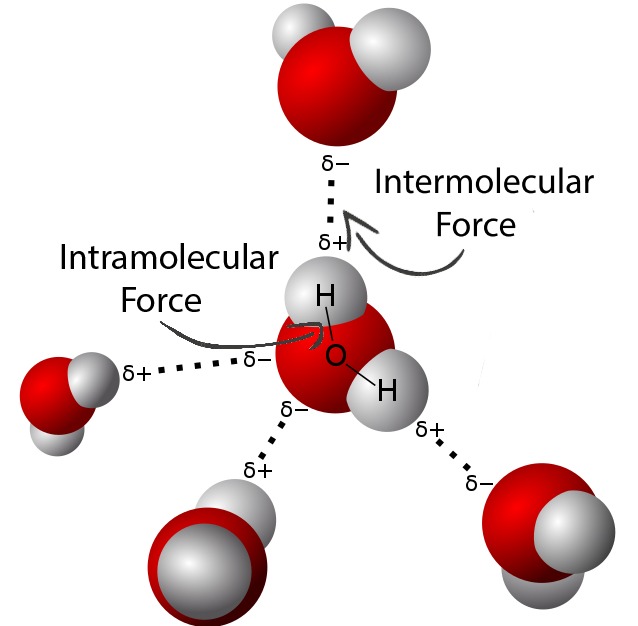
In contrast to intramolecular forces, such as the covalent bonds that hold atoms together in molecules and polyatomic ions, intermolecular forces hold molecules together in a liquid or solid.
Intramolecular forces are strong.
Intermolecular forces are weak.
What are intramolecular and intermolecular forces?
Intermolecular forces are also electrostatic in nature; that is, they arise from the interaction between positively and negatively charged particles.
There are different types of intermolecular forces, including Dipole–Dipole Interactions, London Dispersion Forces, Hydrogen Bonds and ion–dipole interactions.
Intermolecular forces are generally much weaker than covalent bonds. For example, it requires 927 kJ to overcome the intramolecular forces and break both O–H bonds in 1 mol of water, but it takes only about
41 kJ to overcome the intermolecular attractions and convert 1 mol of liquid water to water vapor at 100°C.
Why do intermolecular forces matter?
Intermolecular forces determine properties including the melting points of solids and the boiling points of liquids.
Liquids boil when the molecules have enough thermal energy to overcome the intermolecular attractive forces that hold them together, thereby forming bubbles of vapor within the liquid.
Similarly, solids melt when the molecules acquire enough thermal energy to overcome the intermolecular forces that lock them into place in the solid.
Polar molecules
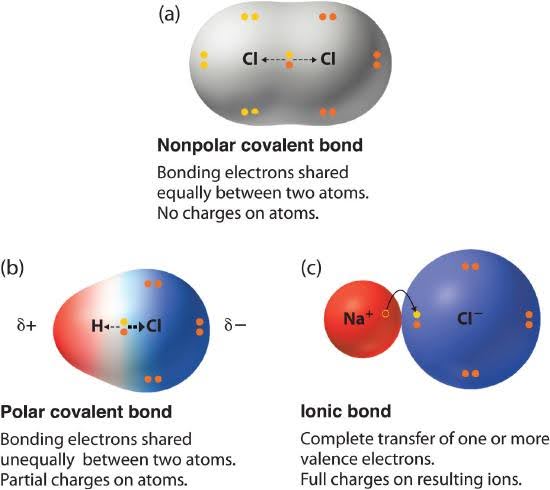
What are polar covalent molecules?
Bond polarity is a useful concept for describing the sharing of electrons between atoms within a covalent bond:
- A nonpolar covalent bond is one in which the electrons are shared equally or nearly equally between two atoms.
- A polar covalent bond is one in which one atom has a greater attraction for the electrons than the other atom.
- If this relative attraction is great enough, then the bond is an ionic bond.
What is unusual about H2O, HF and NH3?
Molecules with hydrogen atoms bonded to atoms such as O, N, and F tend to exhibit unusually strong intermolecular interactions.
These result in much melting and higher boiling points than are observed for other substances as illustrated for the covalent hydrides of elements of groups 15–17.
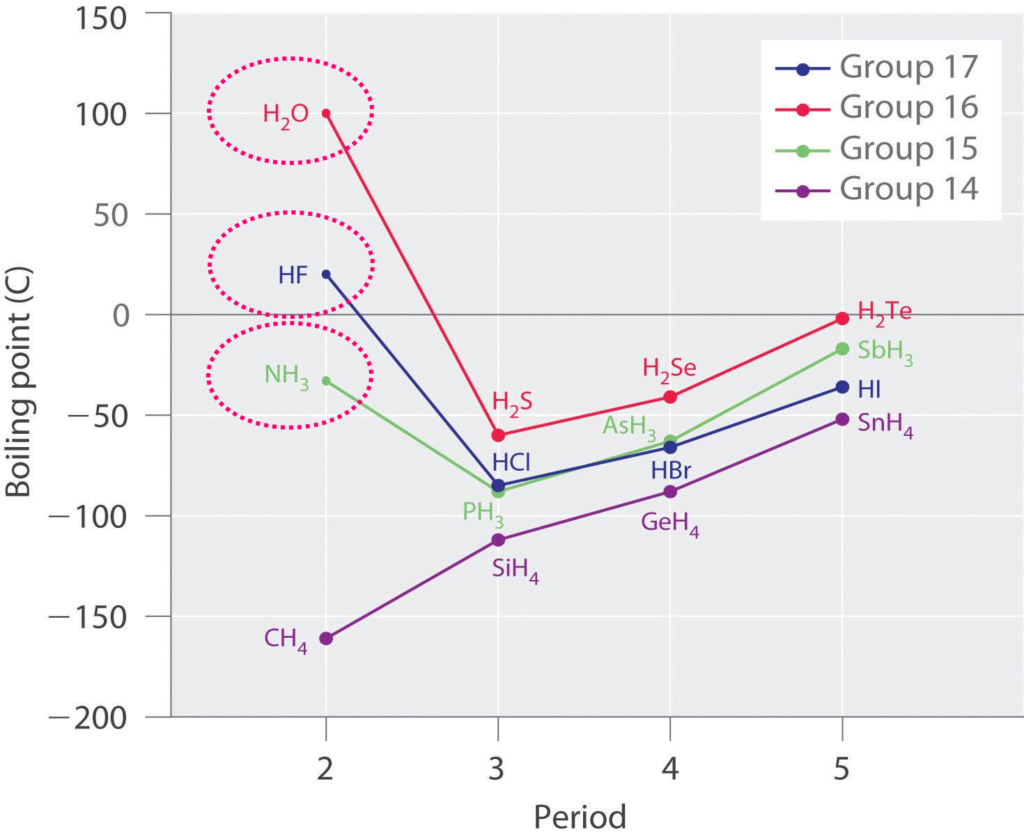
Why is water a highly polar molecule?
The nucleus of an oxygen atom is more attractive to the electrons of hydrogen atoms than the hydrogen nucleus is to the oxygen’s electrons.
We call this attractiveness electronegativity.
Oxygen has a higher electronegativity than hydrogen and the shared electrons spend more time in the vicinity of the oxygen nucleus than they do near the nucleus of the hydrogen atoms.
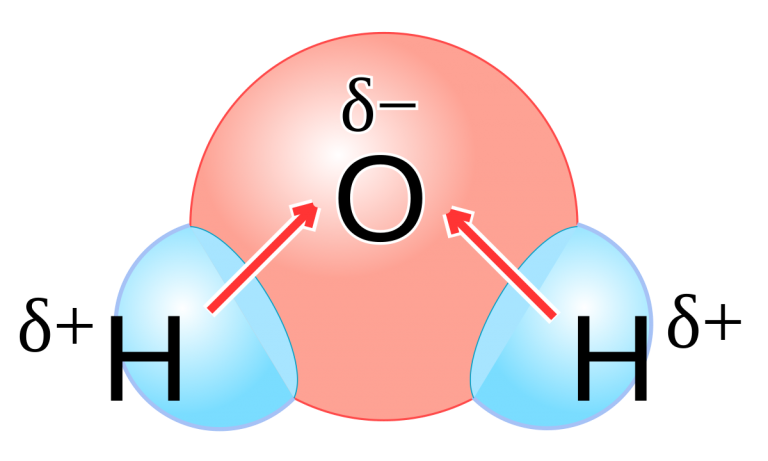
We say that the bonds between hydrogen and very electronegative elements such as O are highly polar.
This gives the atoms of oxygen and hydrogen slightly negative (δ–) and slightly positive (δ+) charges, respectively.
What are the properties of substances made of polar molecules?
Polar molecules (like H2O) have increased attraction between molecules resulting in increased:
- melting points
- boiling points
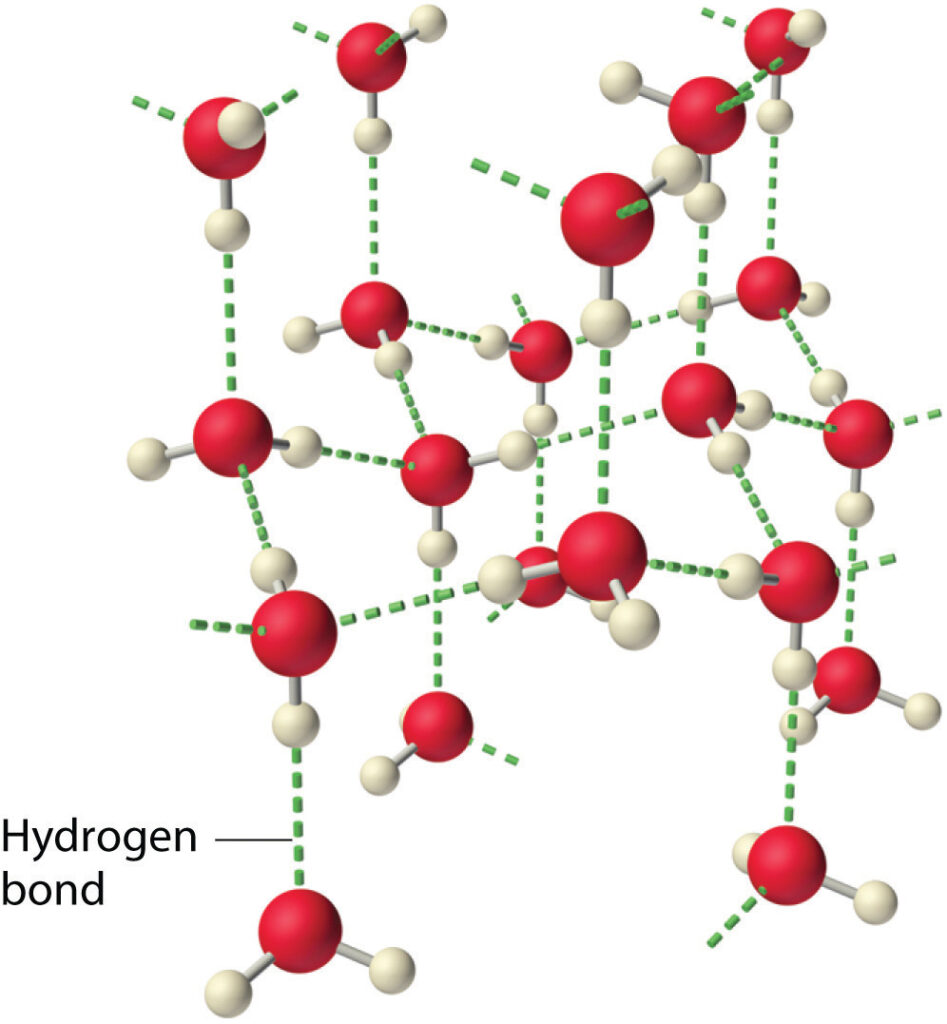
The intermolecular forces in liquid water are among the strongest such forces known! (libretexts 2019).
The intermolecular forces between substances containing H–O, H–N, and H–F bonds are called hydrogen bonds.
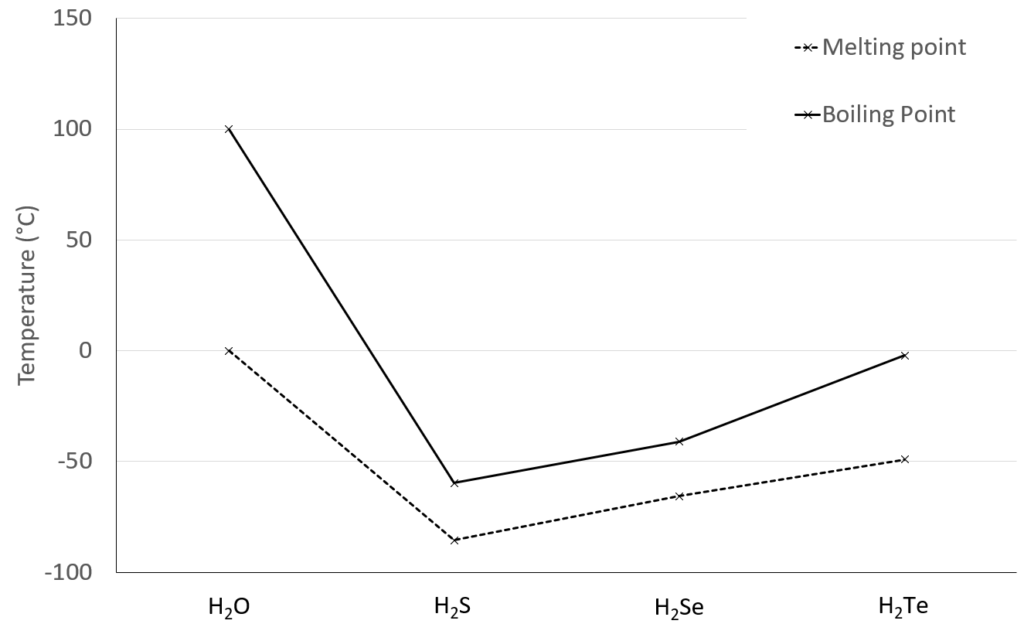
How do the melting & boiling points of water compare?
The melting and boiling points of water (0°C and 100°C) are unusually high compared to other group 16 hydrides (H2S, H2Se and H2Te).
Allotropes of carbon
What are the allotropes of carbon?
You are expected to understand that “elemental carbon exists as a range of allotropes… with significantly different structures and physical properties” (TASC)
Allotropes are structurally different forms of the same element. Carbon allotropes include diamond, graphite and fullerenes.
You must be able to compare and explain the structure and the following physical properties of diamond, graphite and fullerenes: hardness, melting points, electrical conductivity, thermal conductivity. Include both diagrams and a description of the structure of the allotropes.
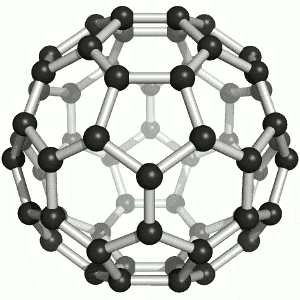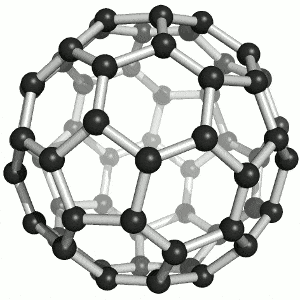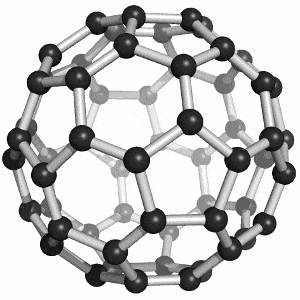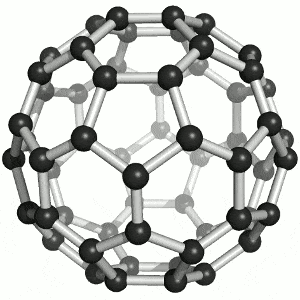
Buckminsterfullerene (C60) By Sponk , CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=11386323
Matching properties to structures
What are the general characteristics of the different structures?
The general characteristics of the different structures are given to you on your information sheet.
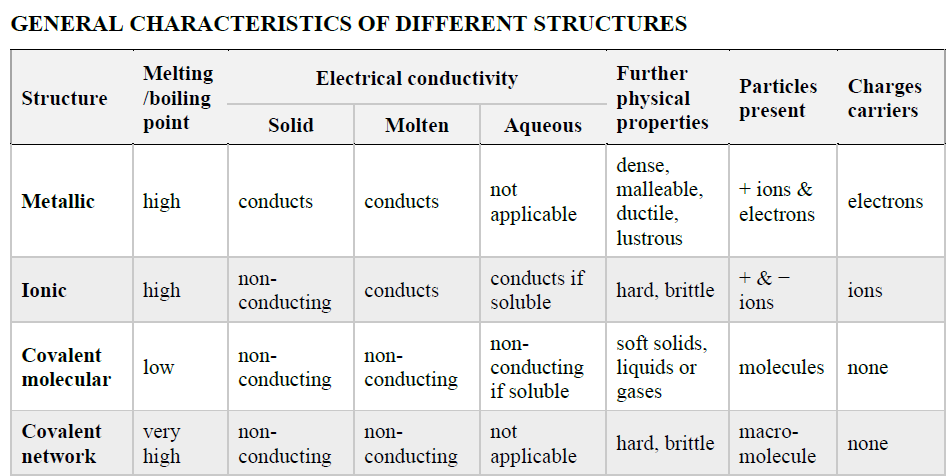
So what sort of questions should you expect? In this course, you are expected to be able to EXPLAIN WHY each of the types of substances has each property (in terms of bonding) and IDENTIFY types of substances based on their properties.