How do we number the groups?
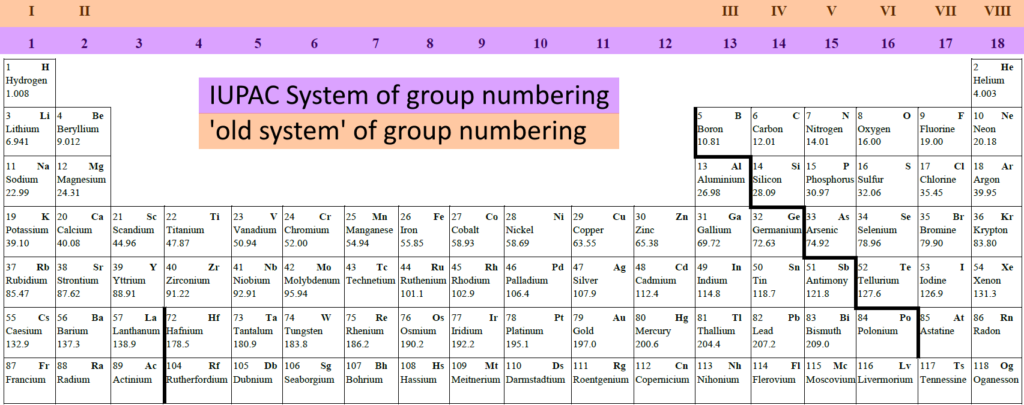
There are two ways to number the groups in the periodic table:
- The IUPAC system uses the numbers 1 to 18 to number the groups containing all elements except the lathanoids and actinoids
- We can also use the ‘old system’ which uses roman numerals I to VIII to number the left and right blocks, not including the transition metals. The advantage of this method is that the group number is the same as the number of electrons in the outer electron shell.
Where are the metals and non-metals?
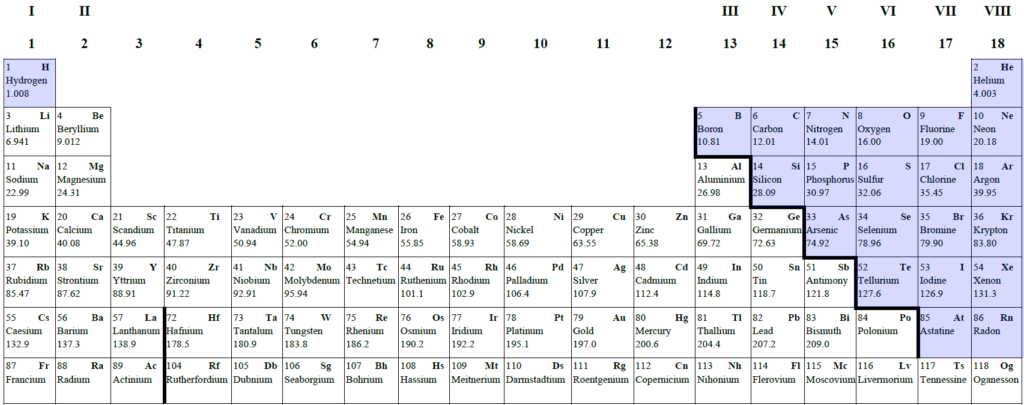
Most of the elements are metals.
The periodic table on your information sheet includes the dividing metal-nonmetal line (the staircase).
It’s a pretty arbitrary division. Even Mendeleev said that ‘It is … impossible to draw a strict line of demarcation between metals and nonmetals, there being many intermediate substances”1
What are diatomic molecules?
The diatomic molecules are simple molecules made of only two atoms.
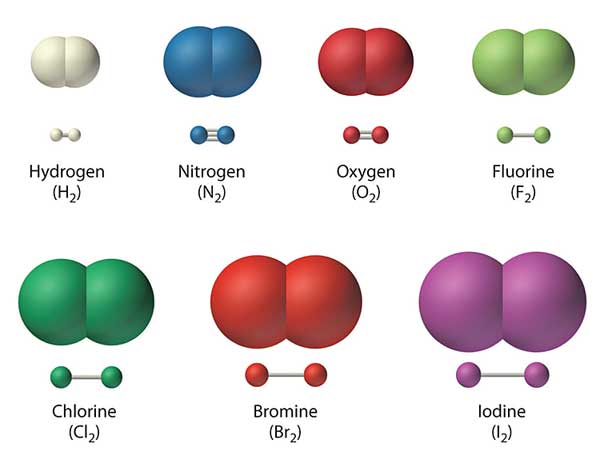
The diatomic molecules can be remembered using the Mnemonics:
- BrINClHOF
Or
- Have No Fear of Ice Cold Beer
All of these elements are gases at room temperature, except for Bromine and Iodine.
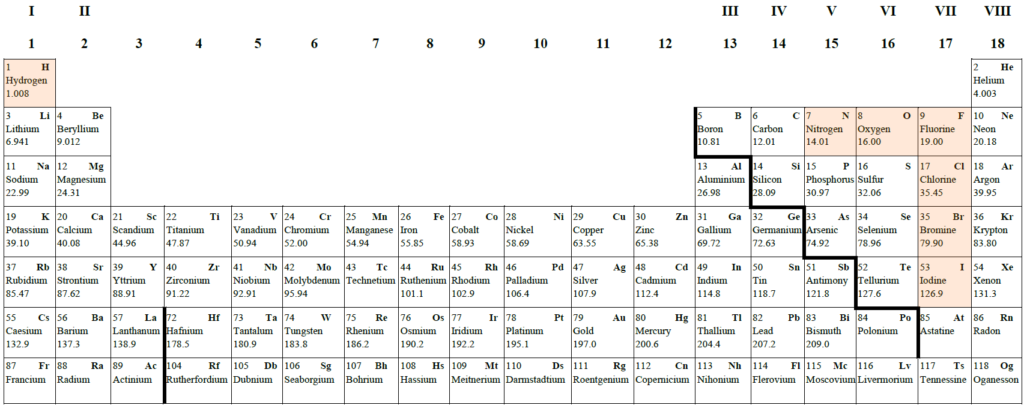
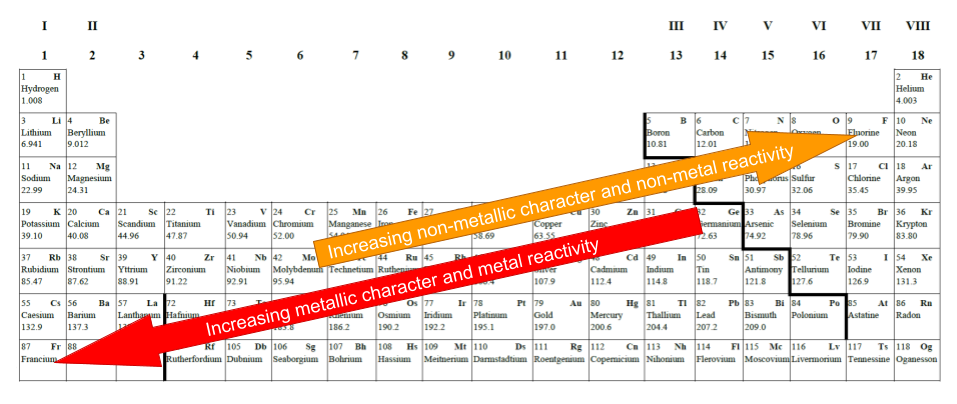
Where are the most metallic elements?
Metallic character can be defined as how easily an element will give away its valence electrons. Thus, metals readily lose their electrons and form positive ions.
Metal reactivity increases as metallic character increases (the metals lose their electrons more readily).
The metallic character (and reactivity) increases as we move down a group
The metallic character (and reactivity) decreases as we move across a group to the right.
What affects metal reactivity?
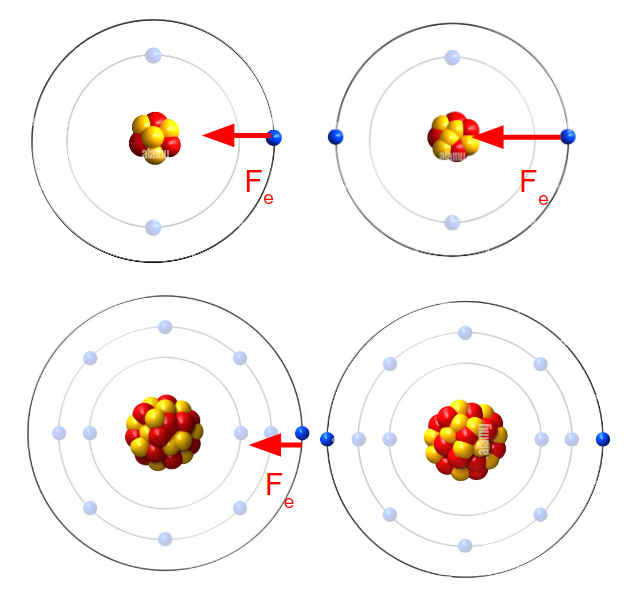
Metal reactivity increases as we move down a group because the valence electrons are more easily lost by the atom because there is less electrostatic force between the electrons and the nucleus because they are further from the nucleus.
Metal reactivity decreases as we move across a group to the right because of the increasing number of protons in the nucleus. As the number of protons in increases, electrostatic force of attraction between the nucleus and the valence electrons increases, so it is harder for the element to lose electrons.
Where are the most non-metallic elements?
Nonmetals tend to gain electrons in chemical reactions, and have a high attraction for electrons within a compound.
The most reactive nonmetals reside in the upper right portion of the periodic table.
The element fluorine is the most reactive nonmetal. It is not found in nature as a free element. Fluorine gas reacts explosively with many other elements and compounds, and is considered to be one of the most dangerous known substances.
Note that there is no clear division between metallic and non-metallic character. As we move across the periodic table, there is an increasing tendency to accept electrons (nonmetallic) and a decrease in the possibility that an atom will give up one or more electrons.
What trends do you need to know?
You are expected to be able to explain reactivity trends in periods 2 and 3 and groups I, II and VII in the periodic table (qualitative).
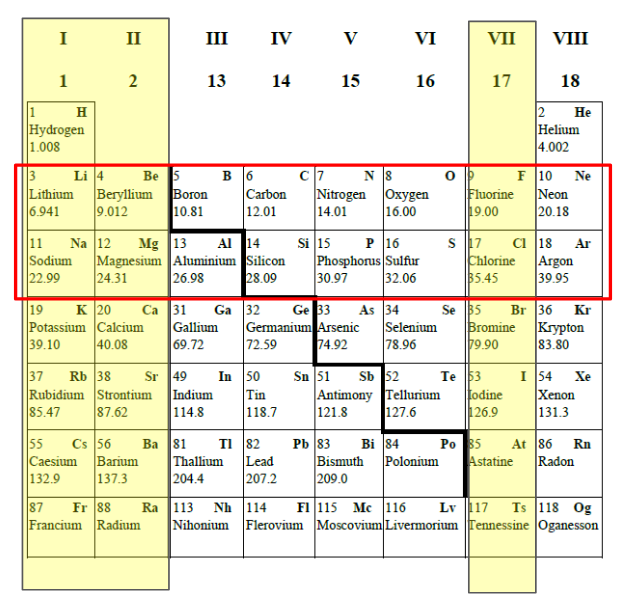
What is the trends in reactivity for groups I and II?
Reacticity increases as we move down the group for both Group I and Group II.

We can explain this trend in terms of the electrons. The elements further down group I and II are more “metallic”. They readily give up their valence electrons. As we move down a group, atoms have increasing numbers of electron shells. The outer electrons are further from the nucleus so there is a reduced force of attraction between them and the nucleus. As a result, less energy is needed for the electrons to be lost.
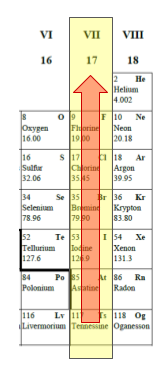
What is the trends in reactivity for group VII?
The reactivity of Group VII elements increases as we move up the group.
We can explain this trend in terms of the electrons. The elements further up the group are more “non-metallic”. The atoms of these elements are more attractive to electrons.
As we move up Group VII, atoms have decreasing numbers of electron shells. The outer electrons are closer to the nucleus so there is an increased force of attraction between them and the nucleus. As a result, the atom is more attractive to electrons.
What is the trend across each Period?

Across each period, from left to right, the increasing attraction between the nuclei and the outermost electrons causes the reactivity to decrease at first.
The reactivity of metals decreases from left to right.
Then, as the nuclear charge increases, and as the elements become more non-metallic, there is increasing attraction between the nucleus and outer shell electrons. The atoms attract electrons more easily.
The reactivity of non-metals increases from left to right (until group VII; remember group VIII elements are unreactive)
What does electron configuration explain?
The ability to form chemical bonds is also known as the reactivity of the element.
“The properties of atoms, including their ability to form chemical bonds, are explained by their electron configurations.” (TASC)
Other properties explained by electron configuration include heat and electrical conductivity.
Decreasing attraction between the nuclei and outermost electrons of metallic elements cause these electrons to be more loosely bound and thus able to conduct heat and electricity.