Concentrations of solutions
How to measure the concentration of a solution?
Concentration can be defined as the amount of solute divided by the volume of solution
Because we can measure the amount of solute in various ways, the concentration of a solution can be represented different ways including:
- the number of moles of the solute per litre of solution (moles per litre, mol L-1) and
- the mass of the solute per litre of solution (grams per litre, g L-1)
How to calculate concentration in moles per litre?
Molar concentration is measured as the number of moles of the solute per litre of solution. It is also known as Molarity.
The unit of measurement for molar concentration is moles per litre, which can be expressed as mol L-1 or sometimes M. Therefore a 1.5 M solution has a concentration of 1.5 mol L-1.
Molar concentration is defined as:
How to calculate concentration in mass per litre?
The other common measurement of concentration used in Physical Sciences is the ratio of the mass of solute to the volume of solvent (e.g. g L–1, mg L–1).
When the concentration unit mg L–1 is required, the mass in grams is converted to mass in milligrams by multiplying by 1000. For smaller solute amounts, mg L–1 is usually more convenient to use.
How to calculate the concentration of ions in solution?
Many ionic substances are soluble. The process of dissolving involves the ionic lattice breaking up and the anions and cations dissociating from each other.
The concentrations of the resulting ions can be calculated. These concentrations are called ionic concentrations and they are designated by square brackets. For example:
[Na+] = 0.5 mol L–1 means that the ionic concentration of Na+ ions in solution is 0.5 mol L–1.
How to solve dilution problems?
When a solution is diluted by the addition of more solvent (e.g. water), the number of moles of solute remains the same. The addition of water to a concentrated solution does not alter the number of moles or the mass of the solute in that solution.
So, if n1 represents the number of moles of the initial or concentrated solution and n2 represents the number of moles of the final or dilute solution, then we can say that:
n1 = n2
Advanced:
Using the equation n = c ✕ V, we can write equations for n1 and n2 as follows:
n1 = c1 ✕ V1 and n2 = c2 ✕ V2
Since the values of n1 and n2 are equal, these equations can be combined to form the following formula:
c1V1 = c2V2
How to convert between mol L-1 and g L-1?
Simply convert the mass to number of moles using
Acids and bases
What are acids?
The definition of acids used in the Physical Sciences course was developed by Johannes Brønsted and Thomas Lowry. It states that:
Acids are proton donors and bases are proton acceptors.
The proton is simply a hydrogen ion, H+.
Most acids encountered in everyday life are aqueous solutions, or can be dissolved in water.
Many acids are molecular substances e.g. hydrogen chloride (HCl) and acetic acid (CH3COOH).
The ionisation (also called dissociation) of nitric acid in water can be represented like this:
HNO3(aq) → H+(aq) + NO3–(aq)
Advanced: What is the hydrogen ion donated to?
The diagram shows the ionization of nitric acid in water.
Note that water functions as a base in this reaction, gaining a proton.
The product of this reaction, the H3O+ ion, is known as the hydronium ion. However, this is not examinable in this course and is often not written in equations when we don’t need to.
In many cases it is perfectly acceptable to use H+ to represent the hydrogen ions and leave out the water, which we will do in this course.
What are Monoprotic and Polyprotic Acids?
“Acids can be classified as monoprotic or polyprotic depending on the number of protons donated by each molecule of the acid”. (TASC).
An acid is said to be Monoprotic if it can donate only one hydrogen ion (H+(aq)) per molecule. E.g. hydrochloric acid, HCl(aq), and nitric acid HNO3(aq)
A Polyprotic acid is able to donate more than one hydrogen ion (H+(aq)) per molecule. The acid can be diprotic if it can donate two hydrogen ions (H+(aq)) per molecule; e.g. H2SO4(aq) (shown) or H2CO3(aq)
How do polyprotic acids ionise?
The ionization for a diprotic acid occurs via two steps. E.g. for carbonic acid:
H2CO3(aq) → H+(aq) + HCO3−(aq)
HCO3−(aq) → H+(aq) + CO32−(aq)
A triprotic acid is able to donate three hydrogen ions (H+(aq)) per molecule; e.g. H3PO4(aq)
NOTE: The ionization for a triprotic acid occurs via three steps; e.g.
H3PO4(aq) → H+(aq) + H2PO4−(aq)
H2PO4−(aq) → H+(aq) + HPO42−(aq)
HPO42−(aq) → H+(aq) + PO43−(aq)
What are the properties of acids?
Acids have many common properties:
- They usually taste sour
- Are corrosive (can damage or destroy other substances with which it comes into contact by means of a chemical reaction).
- Are molecular in structure (but can dissolve in water to produce ions which conducts electricity)
- Affect the colour of certain dyes (they turn litmus, a plant dye, from blue to red)
- Are neutralised by bases
Common acids to memorise (from the TASC Course Description):
- acetic acid or ethanoic acid CH3COOH
- Hydrochloric acid: HCl (the aqueous solution of hydrogen chloride)
- Nitric acid: HNO3
- Sulfuric acid: H2SO4
Citric acid, ascorbic acid and lactic acid are also important examples for biologists.
What are the properties of bases?
Bases have many common properties:
- They usually taste bitter
- Feel slippery to touch (they react with oils in the skin to produce soap)
- Affect the colour of certain dyes (they turn litmus from red to blue and phenolphthalein vivid pink)
- Are generally ionic compounds (if dissolved in water then they are electrolytes that conduct electricity)
- Usually corrosive
A base that is soluble in water is called an alkali.
Common bases that must be memorised:
- Sodium hydroxide – NaOH
- Ammonia, NH3
Oxides of metals are often bases e.g. Calcium oxide CaO
Also carbonates e.g. sodium carbonate Na2CO3
What is the strength of an acid or base?
Depending on the nature of an acid, some or all of its molecules may ionise when the acid is dissolved in water.
The strength of an acid is determined by the extent to which its molecules undergo ionisation.
Stronger acids ionise to a greater extent than weaker acids.
Reactions of acids are often generalized in the form HA ⇌ H+ + A−, where HA represents the acid. This form recognises that the ionisation is a reversible reaction. All reversible reactions reach a point where the forward and reverse reactions take place at the same rate. This point is called dynamic equilibrium. When an acid ionises in water, there is an equilibrium between the acid and the H+ + A− ions.
What are strong acids?
Acids or bases that almost completely ionise in solution are called strong acids or strong bases.
These strong acids share similar properties such as their ability to conduct electricity.
For equations of these, a one-way arrow indicates the reaction proceeds almost entirely in that direction.
Hydrochloric acid, nitric acid and sulfuric acid are strong acids
What are weak acids and bases?
Most acids ionise only to a limited extent in water. Such acids are classified as weak acids.
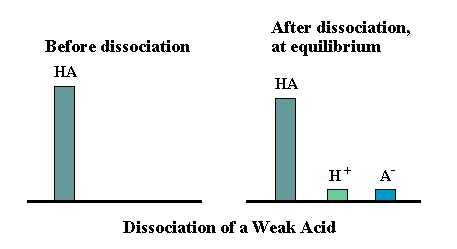
At equilibrium, solutions of weak acids contain a mixture of acid molecules and hydrogen ions.
Acetic acid is a weak acid.
CH3COOH ⇌ CH3COO− + H+
At equilibrium, there will be a mixture of CH3COOH and CH3COO− + H+ ions
What are strong and weak bases?
The concept of strength also applies to bases.
Sodium hydroxide is a strong base. It ionises completely in solution.
Ammonia is a weak base. It ionises only to a small extent in water.
How is strong different to concentrated?
The strength of an acid is different from the concentration of an acid.
The concentration of a solution is defined as the number of particles that are dissolved in a given volume of water. A large amount of a substance dissolved in a given volume is called a concentrated solution, whereas a small amount is referred to as a dilute solution.
The terms strong and weak refer to the degree to which an acid or base ionises in water.
In assessment situations you need to be very careful when using these terms.
The pH scale
What is the pH scale?
The pH scale is a numerical scale used to compare the levels of acidity or alkalinity of aqueous solutions (TASC)
It measurement scale notionally ranging from 1-14 (although very strong acids and bases can be outside these values.)
- Acids have pH < 7
- Bases have pH > 7
- Neutral solutions have a pH = 7 (such as water)
The pH of a solution depends on the concentration of the hydrogen ions in the solution, so dilute hydrochloric acid has a higher pH (e.g. pH = 2) than concentrated hydrochloric acid (e.g. pH = 1).
Advanced: What is pH?
The technical definition of pH = -log10[H+]. You can think of the symbol pH as meaning “power of hydrogen” because the concentration of hydrogen ions, [H+] = 10-pH.
However, for the Physical Sciences course you only need to understand that pH is a measure of the concentration of hydrogen ions in solution.
Because the scale is logarithmic, a solution of pH 2 has 10 times the concentration of hydrogen ions than a solution of pH 3, and 100 times the concentration of hydrogen ions than a solution of pH 4.
For example, stomach acid, a solution of HCl, has a hydrogen ion concentration of
1.2 × 10−3 mol L-1. Its pH is:
pH = -log10[H+] = -log10[H+] = -log10(1.2 × 10−3) = −(−2.92) = 2.93
What is the pH of common acids and bases?
You should be able to identify the pH of some common chemicals:
- HCl
- CH3COOH
- H2SO4
- NH3 and
- NaOH
Reactions of acids
How do acids react with metals?
When an acid reacts with a reactive metal the products are the metal salt and hydrogen.
acid + metal → salt + hydrogen
hydrochloric acid + magnesium → magnesium chloride + hydrogen
2HCl(aq) + Mg(s) → MgCl2(aq) + H2(g)
Remember ionic compounds of metals are referred to as ‘salts’. The course document says “not sulfuric or nitric acids”, so you are likely to be assessed on your understanding of this reaction with hydrochloric acid.
Hydrogen gas can be tested using the pop test.
How do acids react with bases (oxides)?
acid + oxide → salt + water
Example:
sulfuric acid + copper(II) oxide → copper(II) sulfate + water
H2SO4(aq) + CuO(s) → CuSO4((aq) + H2O(l)
This reaction can also be referred to as a neutralisation reaction as the products are neither acidic nor basic.
How do acids react with bases (hydroxides)?
acid + hydroxide → salt + water
Example:
Hydrochloric acid + potassium hydroxide → potassium chloride + water
HCl(aq) + KOH(aq) → KCl(aq) + H2O(l)
This reaction can also be referred to as a neutralisation reaction as the products are neither acidic nor basic.
How do acids react with carbonates?
acid + carbonate → salt + water + carbon dioxide
Example:
hydrochloric acid + copper(II) carbonate → copper(II) chloride + water + carbon dioxide
2HCl(aq) + CuCO3(s) → CuCl2(aq) + H2O(l) + CO2(g)
This reaction can also be referred to as a neutralisation reaction as the products are neither acidic nor basic.
Note that the gas produced, CO2, can be identified using the limewater test.
How do acids react with hydrogen carbonates?
acid + hydrogen carbonate → salt + water + carbon dioxide
Example:
hydrochloric acid + sodium hydrogen carbonate → sodium chloride + water + carbon dioxide
HCl(aq) + NaHCO3 (s) → NaCl(aq) + H2O(l) + CO2(g)
This reaction can also be referred to as a neutralisation reaction as the products are neither acidic nor basic.
Note that the gas produced, CO2, can be identified using the limewater test.
Titrations: identification of unknown inorganic compounds
How to make a standard solution?
Standard solutions are used to determine the concentrations of other substances, such as solutions in titration.
A standard solution is a solution containing a precisely known concentration of an element or a substance. A known mass of solute is dissolved to make a specific volume.
It is prepared using a standard substance, such as a primary standard.
How to dilute a solution?
What is titration?

Acid–base titrations are based on neutralisation reactions.
They are a type of volumetric analysis where the unknown concentration of a solution (the analyte) is determined by reacting it with a solution of known concentration (the titrant).
The titrant is placed in a burette, which is used to deliver definite but variable volumes of liquid. In a titration, the volume of liquid measured by the burette is called a titre.
The analyte is added to a conical flask using a pipette. A pipette is used to deliver a known volume of liquid which is then called an aliquot.
How to perform a titration?
A suitable indicator is added to the aliquot in the conical flask. The solution in the burette is slowly added to the aliquot until the indicator changes colour.
This process is called titration. The point at which chemically equivalent amounts of acid and base (according to the equation) are present is called the equivalence point. The point at which the indicator changes colour is called the end point and is usually about one drop after the equivalence point.
Titres within 0.05 mL of each other are called concordant titres. Three concordant titres are needed to calculate the average titre.