The mole and the Avogadro constant
What is a mole?
The mole (abbreviated: mol) is the SI unit of measurement for amount of substance.
The word ‘mole’ represents a number. It is a unit of measurement, just as the word ‘dozen’ represents 12.
A mole of a substance or a mole of particles is defined as exactly 6.02214076 × 1023 particles, which may be atoms, molecules, ions, or electrons.
A mole was originally defined as the number of atoms in 12 grams of carbon-12 (12C), which has an atomic mass of 12.00 atomic mass units. (It’s now been redefined, but it’s the same number).
Why use the concept of a mole?
It’s useful to understand why a mole equals 6.02214076 × 1023 particles.
Why choose that number?
The Avogadro number was chosen so that the mass of one mole of a chemical compound, in grams, is numerically equal to the average mass of one atom or molecule of the compound, in daltons (or unified atomic mass units, u).
How to calculate the number of moles?
The relationship between the number of moles, n, and the number of particles (atoms or molecules), N, is:
N = n × NA
What is molar mass?
Molar mass is defined as the mass in grams of one mole of a substance. The units of molar mass are grams per mole, abbreviated as g/mol or g mol-1 .
For example, the atomic mass of one atom of neon is 20.18 u, One mole of neon atoms contains 6.02214076 × 1023 molecules, whose total mass is 20.18 g. Thus, the molar mass of neon is 20.18 grams per mole (g/mol).
Molar mass is more commonly applied to molecules than atoms…
How to calculate the molar mass of a substance?
The relationship between molar mass, M, number of moles n and mass m, is:
How to find the molar masses?
The molar mass of an element is simply the same as the relative atomic mass, Ar, expressed in grams per mole.
The information sheet contains a table of relative atomic masses of the elements listed in alphabetical order.
What is the molar molecular mass?
The molar mass of a covalent molecular compound (“molar molecular mass”) is the sum of the molar masses of the atoms making up the molecule (the relative atomic masses).
It’s often called simply the molecular mass.
It’s also given the symbol M and has the units g mol-1.
So CO2 has molar molecular mass, M = 12.01 + (2 × 16.00)
= 44.01 g mol–1.
Thus, 44.01 g of CO2 contains one mole of molecules or 6.02 × 1023 molecules.
What is the relative molecular mass?
The relative molecular mass of a molecule is the average mass of one molecule of that element or compound compared to 1/12 of the mass of one carbon-12 atom.
The symbol for relative molecular mass is Mr.
Like relative atomic mass, Relative molecular mass is a ratio and therefore has no unit.
To calculate the relative molecular mass of a molecule, you need to add the relative atomic masses of all the atoms in its chemical formula.
So CO2 has relative molecular mass, Mr = 12.01 + (2 × 16.00) = 44.01.
The molar molecular mass, M is simply the same as the relative molecular mass, Mr, expressed in grams per mole.
What is the molar mass of an ionic compound?
The molar mass of an ionic compound is the sum of the molar masses of the atoms making up the formula (the relative atomic masses).
It’s often called simply the molar mass.
So NaCl has molar mass 22.99 + 35.45 = 58.44 g mol–1.
What is the percentage composition (by mass) of a compound?
The composition of a compound is often expressed in terms of the percentage that each element contributes to its mass. This is called percentage composition.
For example, potassium makes up 40 % of the mass of potassium chromate.
What are hydrated ionic compounds?
Some ionic compounds crystallise from an aqueous solution to form a hydrated ionic compound.
In these compounds, water molecules are included in the crystal lattice structure.
Hydrated copper (II) sulfate, for example.
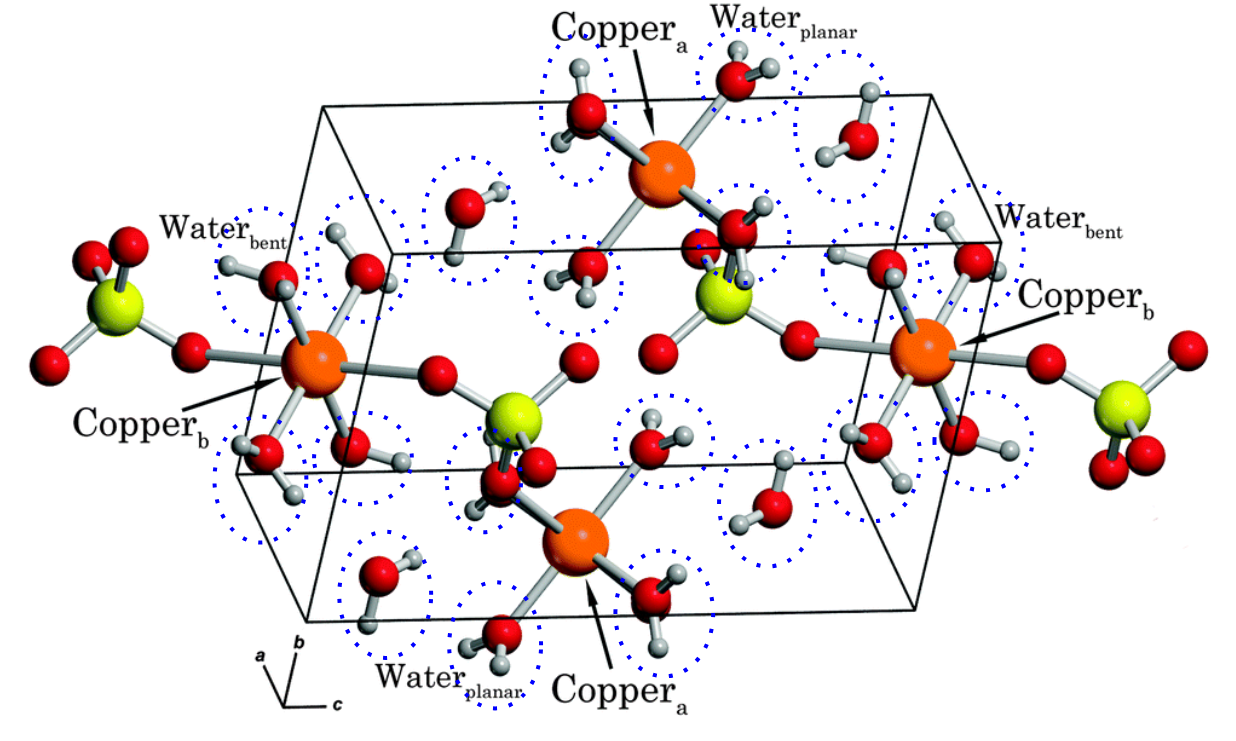
How many water molecules for each copper ion?
What is the water of crystallisation?
The water included in the crystal lattice structure is called water of crystallisation.
Hydrated copper(II) sulfate, for example, appears as a blue crystalline solid and has the formula CuSO4·5H2O.

This means that for each Cu2+ ion and SO42– ion in the crystal lattice structure, five water molecules are also included.
How to determine the mass of water?
Heating the copper (II) sulfate crystals will remove the water molecules to leave a white powder known as anhydrous copper (II) sulfate. The formula of anhydrous copper (II) sulfate is CuSO4.
The mass of the water of crystallisation, and its percentage contribution to the mass of the ionic compound, can be calculated when the masses of both the hydrated and anhydrous compound are known.
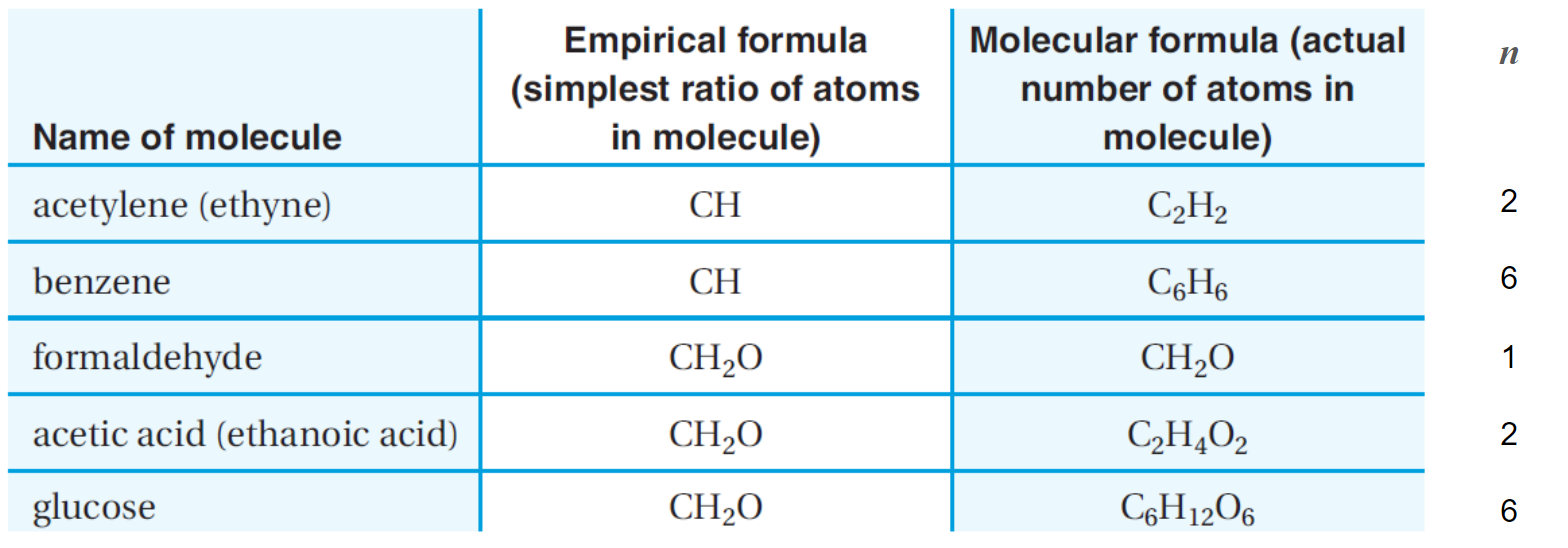
What are empirical formulae?
The empirical formula of a compound is the simplest whole number ratio of atoms of each element in the compound. It is determined using data from experiments and therefore empirical.
For example, the molecular formula of glucose is C6H12O6 but the empirical formula is CH2O. This is because we can divide each number in C6H12O6 by 6 to make a simpler whole number ratio.
What is the empirical formula of C4H8O2?
How to calculate empirical formulae from elemental composition?
- Write down the symbols of the elements present.
- Assume that the mass of the sample is 100 g and all percentages become grams.
- Convert masses to moles.
- Find the simplest whole number ratio of the atoms by dividing all numbers of moles by the smallest number of moles.
- If necessary, multiply by a factor to convert all numbers to whole numbers.
How to determine the empirical formula of a hydrated ionic compound?
The number of moles of the water of crystallisation is always a whole number multiple of the number of multiple of the number of moles of the ionic compound.
E.g. in CuSO4·5H2O, there are 5 moles of water for each mole of CuSO4.
So we can use a similar method to find the empirical formula of hydrated compounds
- Write down the symbols of the ionic compound and water.
- Write down the masses of each.
- Convert masses to moles.
- Find the simplest whole number ratio of the compound by dividing all numbers of moles by the smallest number of moles.
- If necessary, multiply by a factor to convert all numbers to whole numbers.
Molecular formulae
The molecular formula is always some multiple of the empirical formula
molecular formula = n × (empirical formula)
where n represents a whole number
How to determine the molecular formula?
The molecular formula of a compound may be determined from its empirical formula only if its molar molecular mass, Mmolecular is also known.
- Calculate the Empirical Formula mass, Mempirical (also called the “formula mass”).
- Calculate how much larger the Molecular Mass is.
In this case, n is simply the multiplier for the empirical formula
Mass – mass stoichiometry
Why are stoichiometric calculations useful?
Stoichiometric calculations allow chemists to solve problems in which the mass or a reactant or product is given.
Before being inflated, airbags contain a small amount of sodium azide, NaN3. During a collision, an electric spark triggers the decomposition of the sodium azide in the airbag. The reaction produces solid sodium and nitrogen gas:
2NaN3(s) → 2Na(s) + 3N2(g)
The sodium azide reaction must produce the right amount of nitrogen gas to inflate the bag to the correct pressure.
How to solve Mass–mass stoichiometric problems
Mass–mass stoichiometric problems can be solved in four steps:
- Write a balanced chemical equation for the reaction, identifying the known (given) and unknown (required) quantities of substance.
- Calculate the number of moles of the known quantity of substance present.
- From the equation, find the molar ratio that states the proportion of known to unknown quantities in the reaction and use it to calculate the number of moles of the required substance.
- Calculate the quantity (mass) of the required substance.
What is a mole ratio?
N2(g) + 3H2(g) → 2NH3(g)
This equation means that One molecule of nitrogen gas reacts with three molecules of hydrogen gas to form two molecules of ammonia gas.
It also means that one mole of nitrogen gas reacts with three moles of hydrogen gas to form two moles of ammonia gas.
The equation actually indicates the mole ratio of reactants and products.
The beauty of this method is that the ratios never change. If 1 mole of nitrogen reacts with 3 moles of hydrogen, then 2 moles of nitrogen will react with 6 moles of hydrogen, 0.5 mole of nitrogen will react with 1.5 moles of hydrogen, and so on.
A strategy for Sample Mole Ratios Problems
2CH3OH(l) + 3O2(g) → 2CO2(g) + 4H2O(g)
Because the mole ratios for a given chemical reaction never changes, we can equate an unknown ratio to the ratio in the equation.
E.g. How many moles of oxygen are needed to react with 4.0 moles of methanol? The equation ratio = the new ratio so 3O2:2CH3OH = XO2:4CH3OH
In practice, we simply turn the ratios into fractions: